KRAS mutation-positive mucinous adenocarcinoma originating in the thymus
Introduction
Thymic carcinoma is a rare, aggressive disease with a low 5-year survival rate (1-4). Previous report showed that most frequent subtypes were mucinous and papillary subtypes, among 43 cases of thymic adenocarcinoma (5). Primary thymic mucinous adenocarcinoma must be differentiated from metastasis from an extrathymic disease by immunohistochemical findings (5,6).
The detection of KRAS mutation in the mucinous proliferations provides further evidence of their neoplastic nature, and such KRAS positive mucinous adenocarcinoma of the pancreatic, colorectal, and lung have been associated with a more aggressive clinical course (7-9).
Here we describe a case with a KRAS mutation-positive thymic mucinous adenocarcinoma. The diagnosis and treatment of the patient as well as the association between KRAS mutation and prognosis are discussed.
Case presentation
A 39-year-old, previously healthy woman was referred to our hospital because of an abnormality on a chest computed tomography scan. The scan revealed a heterogeneous mass 6.5 cm in size in the anterior mediastinum (Figure 1). The tumor had invaded the left brachiocephalic vein and pericardium. 18F-fluorodeoxyglucose positron emission tomography showed uniform, mild accumulation of 18F-fluorodeoxyglucose (standardized uptake value: 4.77) in the mediastinal mass. The serum level of carbohydrate antigen 19-9 (CA19-9) was 80.5 U/mL (normal: 0–37 U/mL), while the levels of other markers were within the normal range.
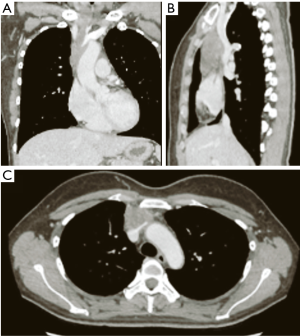
The patient underwent radical resection via median sternotomy following a preoperative clinical diagnosis of thymic carcinoma. During the operation, combined resection of the pericardium, right phrenic nerve, right internal artery, and left brachiocephalic vein was performed owing to tumor invasion. Partial resection and venoplasty with a pericardium patch for the superior vena cava (SVC) were also performed via a temporal bypass between the left brachiocephalic vein and right atrial appendage, using a 6-mm polytetrafluoroethylene graft. The total operation time was 699 min, and amount of blood lost was 702 mL.
Macroscopically, the resected tumor was 6.5 cm × 3.5 cm in size and contained a solid region and multiple cysts with abundant mucin (Figure 2). Microscopic examination showed a papillary growth pattern of goblet cells with round nuclei (Figure 3A). On immunohistochemistry, the resected tumor was positive for caudal type homeobox 2 protein, cytokeratin 7, and cytokeratin 20 (Figure 3B-D), focally positive for CD5, and negative for thyroid transcription factor RNA polymerase I and surfactant apoprotein A; these findings suggest intestinal or ovarian differentiation of the tumor. Subsequent gastrointestinal endoscopy, colonoscopy, abdominopelvic sonography, and imaging analysis did not provide any indication of another primary origin. The serum level of CA19-9 temporarily decreased after the surgery.
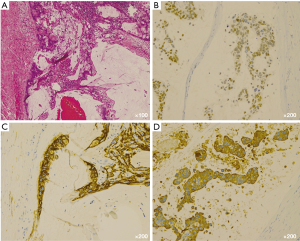
Based on the histopathological and immunohistochemical findings and those of other inspections, the tumor was eventually diagnosed as a mucinous adenocarcinoma of the thymus. It was classified as Masaoka-Koga stage III owing to tumor invasion into the left brachiocephalic vein and pericardium.
Polymerase chain reaction identified a G12V mutation in the KRAS gene in the tumor. There were no mutations in the EGFR gene or EML4-ALK fusion gene. The postoperative course was uneventful, and the patient was discharged on postoperative day 5 while undergoing anticoagulation therapy because of the SVC venoplasty. A year later, multiple lung metastases were detected, and the patient underwent chemotherapy. She is alive 34 months after the initial surgery.
Discussion
Primary thymic adenocarcinomas are exceptionally rare, and the most common subtype is squamous cell (1). Although an evidence-based standard treatment for thymic adenocarcinoma has not been established, surgical resection is typically performed in resectable cases, whereas chemotherapy, radiation, or both may be indicated in unresectable or metastatic cases (2,3). However, complete removal of thymic malignancy is often difficult because the tumor is frequently invades into the surrounding organ and tissue. In current case, adjuvant therapy was not conducted due to patients’ preference, however multiple lung metastases were detected a year after surgery. A recent nationwide cohort study indicated that a significant overall survival benefit with postoperative radiation therapy was found among the patients with thymic malignancies who received chemotherapy (4).
Among the 43 primary thymic adenocarcinomas examined in a recent study, most were the mucinous or papillary subtype (5). Mucinous adenocarcinoma of the thymus resembles mucinous adenocarcinoma of the gastrointestinal tract, pancreas, breast, lung, and ovary. The carcinoma cells contain abundant amounts of mucin in their cytoplasm and often assume a goblet cell or signet ring cell morphology. In cases of mucinous thymic adenocarcinomas, it is necessary to exclude metastasis from other organs (6). Although various immunoprofiles for thymic adenocarcinomas have been reported, the immunohistochemical findings in the study by Moser et al. (5) were the same as those described here. However, no previous studies reported KRAS gene mutations in mucinous thymic adenocarcinomas.
KRAS mutations are particularly common in pancreatic, colorectal, and lung cancers. In addition, several studies have shown that tumors with KRAS mutations have a worse prognosis than do those without KRAS mutations (7-9). KRAS mutations may indicate tumor aggressiveness in thymic carcinomas as well, and further studies should be performed to examine this possibility. Determination of the clinical significance of KRAS mutations in mucinous adenocarcinomas originating in the thymus will further our understanding of their behavior and may aid in their detection and treatment.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflict of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. [Crossref] [PubMed]
- Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303. [Crossref] [PubMed]
- Momozane T, Inoue M, Shintani Y, Funaki S, et al. Trimodality Therapy for an Advanced Thymic Carcinoma With Both Aorta and Vena Cava Invasion. Ann Thorac Surg 2016;102:e139-41. [Crossref] [PubMed]
- Boothe D, Orton A, Thorpe C, et al. Postoperative Radiotherapy in Locally Invasive Malignancies of the Thymus: Patterns of Care and Survival. J Thorac Oncol 2016;11:2218-26. [Crossref] [PubMed]
- Moser B, Schiefer AI, Janik S, et al. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol 2015;39:541-8. [Crossref] [PubMed]
- Seki E, Aoyama K, Ueda M, et al. Mucinous adenocarcinoma of the thymus: a case report. J Thorac Oncol 2008;3:935-7. [Crossref] [PubMed]
- Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92:131-9. [Crossref] [PubMed]
- Guin S, Ru Y, Wynes MW, et al. Contributions of KRAS and RAL in non-small-cell lung cancer growth and progression. J Thorac Oncol 2013;8:1492-501. [Crossref] [PubMed]
- Abubaker J, Bavi P, Al-Haqawi W, et al. Prognostic significance of alterations in KRAS isoforms KRAS-4A/4B and KRAS mutations in colorectal carcinoma. J Pathol 2009;219:435-45. [Crossref] [PubMed]



