The technique of endoscopic airway tumor treatment
Introduction
Lung cancer accounts for about 13% of all cancers diagnosed in Europe (1), with approximately 400,000 new diagnoses each year. More than half of primary lung cancers are not resectable at diagnosis and 40% of deaths may be secondary to loco-regional disease. Many of these patients suffer from symptoms related to airways obstruction (respiratory distress, bleeding, atelectasis, post-obstructive pneumonia, etc.).
Epidemiology, characteristics and treatment indication in central airway tumors
According to one estimate, 20–30% of newly diagnosed patients with lung cancer will develop complications associated with partial or complete airway obstruction (2).
Central airways obstructions (CAO) are secondary to several malignant and non-malignant diseases such as primary lung cancer, central airways tumors (relatively rare, 600–700 cases per year) or benign tracheobronchial tumors. In addition, airways can present early-stage cancer like carcinoma in situ (CIS) or minimally invasive lesions. In patients with this complication, morbidity is significant. If left untreated, death from suffocation is a frequent outcome. It is obvious that in cases with life threatening obstruction of airways there is an urgent need for rapid re-canalization. Bronchoscopic local treatment is an easy to perform procedure, less invasive than surgery and more immediate than radio/chemotherapy. It is quick and does not interfere with possible future surgical resection in case of treatment failure.
Indications for therapeutic endoscopic treatment are palliation of dyspnea and other obstructive symptoms in advanced cancerous lesions and cure of early lung cancer. Clinicians should select cases, evaluating tumor features (size, location) and patient characteristics (age, lung function impairment) to choose the most appropriate endoscopic technique.
Indeed, although indications for endoscopic treatments were mainly for palliation of advanced endobronchial cancerous lesions, there is increased attention devoted to adjuvant therapy and even cure of early lung cancer.
Benign tracheobronchial tumors
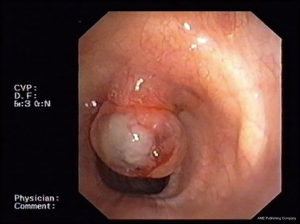
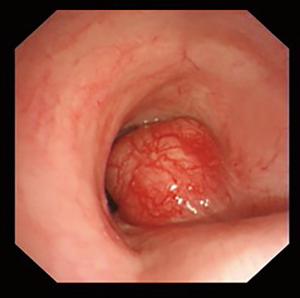
Benign tracheobronchial tumors are rare (around 2% of all lung cancer) and difficult to diagnose (Figure 1). Their clinical and radiographic features are non-specific and often go unrecognized for a long time. Main symptoms are related to bronchial obstruction and typical radiographic findings are atelectasis, obstructive pneumonia and mediastinal shifts (3). Benign tumors have usually a good patient outcome and providing an early diagnosis allows to treat them conservatively (4) (Figure 1).
CT scan is a crucial first step in surgical or operative bronchoscopy planning because it provides information about tumor size and extent or evidence of adjacent mediastinal and parenchymal invasion (3). All these morphological features can also be assessed using endobronchial ultrasounds (EBUS) (5). Patients who suffer from airway obstruction secondary to benign tumors are candidates to surgery (6). Anyway, surgery is difficult to perform on large airways (trachea, main bronchi) (7) and bronchoscopic management plays a key role in removing the obstruction and recovering ventilation (8). The most common bronchoscopic techniques to treat benign tracheobronchial tumor are thermal coagulation using electrocautery (9), argon plasma coagulation (10) or neodymium-doped yttrium-aluminium-garnet (Nd:YAG) laser (11,12) and cryotherapy (13,14).
These techniques are indicated to treat endoluminal tumors that do not exceed subsegmental bronchi, with small base of implant (<15 mm2) and without any evidence of submucosal-layer infiltration. Tumors with peduncular structure (polypoid) are the most suitable to undergo endoscopic resection. Bronchoscopic management is also indicated for all those patients not suitable for surgery due to their clinical conditions. When correctly performed, all the techniques mentioned above permit to avoid recurrence and future surgery.
CIS or minimally invasive endobronchial lesions
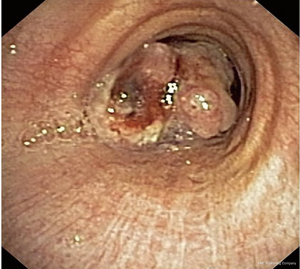
Early-stage lung cancer is a pre-invasive or minimally invasive asymptomatic lesion often diagnosed by chance (15) (Figure 2). Surgical approach is currently still the gold standard for the treatment of this tumor and 5 year survival rate is pretty high (80–90%) (16-18). However, surgery presents some disadvantages, first of all, to be curative often needs a large resection of normal lung parenchyma (from lobectomy to pneumonectomy), impacting negatively on patients quality of life. Furthermore, synchronous and metachronous lesions can be detected in smokers airways (20% and 14–30% respectively in central SCC) (16-19).
About CIS or slightly invasive endobronchial lesions, interventional pulmonology offers a quick, safe and effective treatment with good results, low morbidity and low cost (20) even if compared with radiotherapy (21). Furthermore, bronchoscopy allows to avoid surgery in many patients with impaired lung function or poor clinical status. Different bronchoscopic techniques have been used to treat pre-invasive or minimally invasive lesions with curative purpose (16,22). They should be applied to flat-type squamous cell carcinoma (SqCC) in situ and microinvasive cancer extended less than 10 mm with visible tumor margins and no evidence of extra-cartilaginous invasion (16). The treatment success depends on accurate staging (23). Tumor extension can be evaluated with autofluorescence bronchoscopy (AFB) examination and wall invasion can be accurately excluded by EBUS, while nodal involvement can be assessed by PET. An incorrect assessment of disease extension and nodal involvement is the main cause of treatment failure (23).
Various bronchoscopic techniques can be used: thermal coagulation (electrocautery, argon plasma coagulation or Nd:YAG), photodynamic therapy (PDT) and cryotherapy. In early lung cancer, endoscopically treated, the cure rate is between 43% and 97% but recurrence rate is quite high and a significant number of lesions require a second treatment to obtain a definitive cure (24) (Figure 2).
A specific discussion should be reserved to bronchial carcinoid, a relatively rare, minimally invasive, neuroendocrine neoplasm (Figure 3). Potential and limitations of the endoscopic approach should be considered in deep. Bronchial carcinoids, in fact, usually origin in the central airways and typically produce symptoms and signs associated with airway obstruction (i.e., cough, wheezing and other asthma-like signs, etc.), recurrent pneumonias or hemoptysis due to the tumor hypervascularization.
Tumor removal is considered the treatment of choice and should be pursued by methods minimizing the procedural stress (25). Conservative lung sparing surgery, such as sleeve resection or similar, is currently considered the elective therapy for peripheral lesions (6); on the other hand, in centrally located carcinoids, surgery is often technically demanding for the surgeon and detrimental to the patient (26).
Indeed, growing evidence is now documenting that endobronchial treatment may be considered, in selected cases, a safe and effective treatment for typical carcinoid tumors in the central airways (27). Furthermore, the addition of initial endobronchial treatment has no negative effect on the surgical outcome (28).
Based on individual expertise and good sense, two different endoscopic approaches should be evaluated:
- Symptomatic relief or pre-surgical treatment: to be considered in broad based (flat, non-polypoid, without a clear stalk or with a wide, not pedunculatus one) tumors, not extending across multiple cartilaginous rings and invading extramural tissues (29). The symptomatic endoscopic approach aims, as in common central airways malignant tumors, to restore bronchial patency allowing peripheral ventilation and allow the drainage of post stenotic infected endobronchial material (30). The pre-surgical tumor eradication is useful to warrant a detailed evaluation and location of the base of the tumor in order to plan a lung sparing surgery (31).
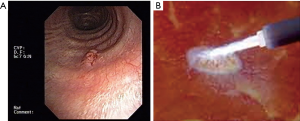
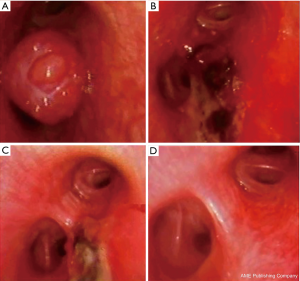
- Eradication treatment: the endoscopic approach with curative intent, is amenable only for typical carcinoids, since this subgroup carries an excellent prognosis (87–100% 5 year survival) and a low incidence of nodal invasion (5–20%) (32). The only independent factor associated with a recurrence of the typical carcinoid and a consequent poorer prognosis is uncompleted resection. The likelihood of endoscopic successful eradication is strictly related to the neoplasm characters (centrally located, totally endobronchial, with a small basal implant of less than 1–2 cm2, non-infiltrating the submucosa), to the appropriateness of the resection technique and to the proper laser treatment of the tumor’s base. When all of these standards are warranted the endoscopic treatment can realistically be considered an alternative to open surgery (33). Indeed, the final indication of the successful eradication after the endoscopic approach can be achieved at a 30–60 days bronchoscopic control and after a total body CT scan is performed to confirm the absence of extrabronchial involvement or mediastinal adenopathies. This time lapse is required to allow the treated mucosa to recover and scars to heal. Furthermore, the absence of blood, will allow the laser assisted treatment of the tumor’s implant that is not possible in presence of tissue hyperpigmentation (as it occurs during bleeding) that limits the laser’s penetration in deep and decreases its “cytocide” effect (34) (Figure 4).
Malignant central-airway obstruction
Epidemiology shows that about 20–30% of patients affected by lung cancer suffer from symptoms related to CAO (35). In addition, numerous solid tumors (2%) can lead to malignant airway obstruction (MAO) through compression/infiltration (esophagus, thyroid) or metastatic involvement (kidney, colon, breast and melanoma). CAO may occur in three main ways: intraluminal tumor growth, airway compression secondary to an extraluminal mass or a combination of these two processes (Figure 5).
Rigid bronchoscopy is a valuable tool to treat malignant obstructions because ensures airway control and the possibility to remove large volumes of tumor or to place stents. The decision to perform rigid bronchoscopy should be undertaken after a full evaluation of available therapeutic strategies (including chemotherapy and radiations) and their success rate.
Depending on mechanisms of stenosis, various bronchoscopic techniques can be chosen and performed alone or combined (36). If the pathogenic process is driven by an intraluminal tumor growth, the operator can use mechanical debulking or thermal techniques. Thermal techniques are further divided upon their immediate [laser, electrocoagulation, argon-plasma coagulation (APC)] or delayed effect (cryotherapy, PDT). An immediate effect is necessary to treat severe or very symptomatic stenosis; a delayed effect can be used on non-threatening stenosis. When central airway obstruction is secondary to an extrinsic compression or mixed obstruction, an airway stent (silicone or metallic) can be placed (37). Indications to an invasive treatment are symptomatic obstruction associated to a viable bronchial tree and normal parenchyma. These findings have to be confirmed before perform interventional bronchoscopy, using CT-scan and flexible bronchoscopy. The aims of all these techniques are palliation of the dyspnea, control of the hemoptysis and contribution to treat obstructive pneumonia. Although, treating large airways tumors gives best success rate (38).
Clinical and radiological presentation
The clinical presentation of central airways tumors is usually nonspecific. Dyspnea is the main symptom and is obviously shared by many different conditions. Other manifestations include hemoptysis, wheezing, cough, hoarseness of voice, limited exercise capacity, and reduction of lung function parameters (35). Delayed diagnosis is frequent, because these symptoms could raise a false diagnosis of other diseases such as asthma, COPD and pneumonia. Onset and nature of symptoms are largely dependent upon tumor size, anatomic location, and contact with surrounding structures. Generally, symptoms of airflow obstruction are mild to moderate for a long time, until the airway lumen becomes critically reduced. In this situation a quick intervention to restore airflow is demanded (39).
Even if conventional radiography is able to identify a centrally located tumor in up to 30% of the cases (40), computed tomography of the chest is mandatory, given its ability to detect tumor, size, local extension, and invasion of adjacent structures. CT scan can also localize distant metastases. In addition, multidetector CT (MDCT) gives the possibility to perform post-processing techniques like multiplanar reformation (MPR), volume rendering (VR) and virtual bronchoscopy (VB). These techniques could add morphological informations regarding tumor location and shape, extramural invasion, longitudinal spread, and distance between bronchial tumors and main carina (41).
Preoperative assessment
Collaboration between the anesthetist and the bronchologist is critical for the endoscopic treatment of airway lesions. General anesthesia is necessary for the endoscopic treatment of neoplastic lesions, since this procedure is carried out with a rigid bronchoscope in the majority of cases, and ancillary techniques like laser therapy, cryotherapy or stent implantation are often employed. The preoperative assessment is therefore important for narrowing the risk of major complications. A detailed evaluation should cover comorbidities, cardiovascular efficiency, ventilatory function, anatomical localization of the tumor, coagulation parameters, and arterial blood gas (ABG). Patients requiring endobronchial treatment could have impaired cardio-pulmonary reserve, pulmonary hypertension and severe obstructive and/or restrictive functional defects (42). It is unclear if routine preoperative pulmonary function testing is mandatory, given its limited role in prediction of post-operative complications (43). Nonetheless, any potentially reversible or ameliorable lung function limitation should be treated and minimized before endoscopy. Usually, patients suffering from neoplastic airflow obstruction could remarkably benefit from endoscopic treatment, as measured by exercise capacity, spirometric parameters, dyspnea- specific and overall quality of life scores (44). Anyway, endoscopic treatment is generally not indicated in patients with a short life expectancy (less than 3 months). An informed consent should be obtained for both endoscopic procedures and anesthesia.
Diagnosis and endoscopic assessment of central lung cancer and preinvasive lesions
Bronchoscopy is the standard imaging tool for the diagnosis of central airway lung cancer (45). However, this technique is limited in its ability to detect small early central cancers and preinvasive lesions of the airway (46). Bronchial intraepithelial lesions may be precursors of central airway lung carcinomas. Indeed, central airway carcinomas are considered to develop gradually from preinvasive epithelial lesions (47). In particular, SqCC which is the second most frequent type of lung cancer, in contrast to adenocarcinoma, is believed to arise in the central airways through a stepwise series of molecular and cellular alterations in which the airway epithelium progresses from normal to hyperplasia, metaplasia, dysplasia (mild, moderate, and severe), and finally CIS (47). In general, dysplasia (in particular severe forms) and CIS are considered the most important preinvasive lesions for SqCC. In dysplasia the cellular changes extend to the entire airway epithelium but without reaching the surface. Lesions that progress to CIS show extreme cytologic aberration that extend throughout the airway epithelium but do not infiltrate the basement membrane (48). According to an alternative model, multiple foci of precursor lesions are produced throughout the respiratory epithelium as a consequence of exposure to smoking. SqCC may develop from any of these lesions rather than because of stepwise progression of a single area (49). Early detection and follow-up of these pre-malignant lesions are essential because they have a high chance of progressing to invasive cancer (50). To detect these smaller lesions, emerging techniques have been proposed in the last decades as new imaging tools integrating the standard white light bronchoscopy (WLB). They are AFB, narrow band imaging (NBI) and high-definition bronchoscopy.
AFB
AFB is an advanced technology that exploits the autofluorescent nature of bronchial mucosa to detect tiny and subtle superficial lesions (51). Several types of AFB systems have been designed, developed, and marketed, including the LIFE system (Xillix Technologies, Richmond, British Columbia, Canada), the D-Light system (Karl Storz, Tuttlingen, Germany), and the SAFE system (Pentax, Tokyo, Japan). Finally, autofluorescence imaging (AFI) bronchovideoscope system (Olympus Optical Corporation, Tokyo, Japan) is a newly developed AFB system that consists of three parts: an autofluorescence videobronchoscope, a video processor unit and a xenon light source (Figure 6).
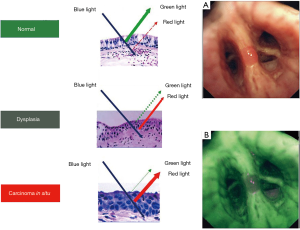
Autofluorescence is intensely produced by submucosal stroma, but epithelium, mucosa, and cancerous tissue emit very little fluorescence. Several mechanisms have been hypothesized to explain the different autofluorescence of normal and cancerous tissues: for example autofluorescence can change if the epithelial layer thickens, as in the presence of cancer, or if the concentrations of certain substances in the tissue, including fluorophores and nonfluorescent chromophores, change (51). Most endogenous fluorophores are associated with the tissue matrix, such as collagen and elastin, or are involved in cellular metabolic processes. One of the major causes for the loss of autofluorescence in areas of dysplasia or cancer is found to be a decrease in the extracellular matrix content (52). The intensity of the autofluorescence differs substantially between normal and tumorous tissues, which allows visualization of cancers and precancerous lesions in bronchi. According to the different systems, normal bronchial mucosa appears green or gray, while cancers and pre-invasive lesions appear brown, or brown-red and dark.
Several studies have reported the diagnostic performance of AFB and most have concluded that AFB has a much higher sensitivity and a lower specificity than WLB in detecting precancerous lesions (53-57). Indeed AFB provides many false positives (such as inflammation, infection or trauma), due to the high blood flow and metabolism that increase light absorption, diminishing green autofluorescence in tissue (58). The role of AFI for the early detection of preinvasive bronchial lesions in comparison with WLB and LIFE has been reported by Chiyo et al. (59). The sensitivities for dysplasia by AFI, LIFE and WLB were 80%, 96.7% and 53.3%, respectively, while the specificities were 83.3% for AFI, 36.6% for LIFE and 50% for WLB (59).
Similarly, three meta-analyses demonstrated that AFB and AFB + WLB have more advantages than WLB alone for detection of lung cancer and precancerous lesions, showing a higher sensitivity but a lower specificity. In the meta-analysis by Sun et al., 21 studies involving 3,266 patients were analyzed (60). The pool relative sensitivity of AFB + WLB versus WLB alone to detect intraepithelial neoplasia and invasive cancer was 2.04 and 1.15, respectively. The pool relative specificity of AFB + WLB versus WLB alone was 0.65 (60). Chen et al. included in their meta-analysis 14 studies, providing 15 sets of data (61). The pooled sensitivity and specificity of AFB and WLB were 0.90 and 0.56, 0.66 and 0.69, respectively (61). Finally, in the most recent meta-analysis, 39 studies using WLB, 39 using AFB and 17 using AFB + WLB were selected (62). Sensitivity and specificity were 54% and 79% for WLB, 87% and 65% for AFB, and 88% and 59% for AFB + WLB, respectively. In the same study, the diagnostic performances of the two techniques were calculated selectively for high-grade lesions from moderate dysplasia to invasive carcinoma. Sensitivity of AFB + WLB was similar to that obtained in the entire data set (86%) but specificity increased to 71% (62).
Another important contribute provided by AFB is the assessment of tumor extent and margins. Zaric et al. found that AFI videobronchoscopy system yields significantly higher sensitivity and specificity for the assessment of lung cancer extent than WLB videobronchoscopy alone (63). In this way this technology can affect therapeutic strategy in central lung cancer.
In conclusion AFB used together WLB seems to significantly improve the sensitivity to detect intraepithelial neoplasia compared with WLB alone and could be valuable to avoid missed diagnosis. However, this higher sensitivity is achieved at the expense of a lower specificity. Thus, use of AFB may also result in a higher number of unnecessary biopsies. Moreover, in evaluating the extension of central lung cancer, AFB is able to influence therapeutic option for lung cancer treatment.
NBI
NBI is a new optical technology that can clearly visualize the microvascular structure on mucosal surfaces (64). NBI uses a narrow banding filter which cuts all wavelengths in illumination except for narrow bands in the blue and green spectrum, centered at 415 and 540 nm, coinciding with the peak absorption spectrum of oxyhemoglobin (the main chromophore in bronchial tissues), making blood vessels more pronounced (65). On the basis of the characteristics of the vascular network of the bronchial wall, NBI allows the differentiation of dysplasia, CIS, micro-invasive tumour and invasive SqCC (Figure 7).

In a prospective trial that enrolled 96 patients with indication for bronchoscopy, combination of AFI and NBI was evaluated in detection of premalignant bronchial lesions (66). Combination of NBI and AFI significantly improved sensitivity when compared to each individual technique (P<0.001). When specificity is of concern, combination of techniques improved specificity of WLB (P<0.001) and specificity of AFI (P=0.03), but it does not have significant influence on specificity of NBI (P=0.53). Thus, combination of NBI and AFI increases both sensitivity and specificity of each technique but it seems that NBI is most sufficient and effective in detection of premalignant bronchial lesions. In another study, the diagnostic yields of NBI individually and in combination with WLB and AFI has been evaluated in early lung cancer (67). Fifty-seven patients who were referred for airway screening or surveillance had a 30% prevalence of intraepithelial neoplasia. Both NBI and AFI had superior sensitivities, compared with WLB alone, and there was no significant difference in sensitivity between NBI and AFI. There was no significant difference in specificity between NBI and WLB, but AFI’s specificity was significantly lower than either NBI or WLB. The authors concluded that NBI is an alternative to AFI in the detection of early lung cancers because it has a comparatively higher specificity without significantly compromising the sensitivity (67).
A recent meta-analysis assessed the diagnostic performance of NBI alone and combined NBI + AFI (68). The results suggested that NBI is better than AFI in the detection of premalignant airway lesions, showing a pooled sensitivity and specificity of 80% and 84%, respectively. The combination of AFI and NBI did not significantly improve the diagnostic performance, yielding a pooled sensitivity and specificity of 86% and 75%, respectively. In another meta-analysis which included six studies, NBI presented remarkable diagnostic performance with a 96% sensitivity and 84% specificity (62). However the diagnostic performance of NBI was lower for the high-grade lesions from moderate dysplasia to invasive carcinoma since the specificity decreased to 43% (62).
The combination of AFI and NBI has been also tested for the evaluation of extension of central lung cancer. A prospective study in 118 patients with suspected lung cancer reported that the combination of techniques showed significantly better sensitivity and specificity in the assessment of lung cancer extension when compared to WLB alone, but improvement was not so convincing when compared to the each technique alone (69).
In conclusion, although the role of NBI for detection of premalignant airway lesions and early lung cancer has been largely studied and confirmed, further comparative data between NBI and AFI are still needed to draw conclusions on the superiority of one technique over the other (51,58).
Other developing techniques
Other new technologies have been developed with the aim to better visualize the bronchial vascular tree. Vascular changes have been shown to correlate to angiogenic squamous metaplasia and lung adenocarcinoma or squamous carcinoma, as was reported in studies using the NBI (70-72). Therefore, facilitation of detection of these subtle changes is of clinical importance. In a recent study van der Heijden et al. aimed to explore the sensitivity of high definition (HD) images with advanced real time image enhancement techniques (i-scan) bronchoscopy for detection of epithelial changes like vascular abnormalities and suspicious preinvasive lesions, and tumors (73). Vascular abnormalities were scored most frequently in HD + i-scan bronchoscopy as compared to AFB (P=0.003). Sites suspicious for preinvasive lesions were most frequently reported using AFB as compared to both WLB and HD bronchoscopy (P=0.003). The authors concluded that HD-bronchoscopy with i-scan technique may result in better detection of subtle vascular abnormalities in the airways which in turn may be related to preneoplastic lesions and tumors (73).
Another similar technique is the high magnification bronchovideoscopy (HMB) which is able to observe vascular networks of the bronchial mucosa (74). Shibuya et al. found that HMB could detect dysplasia more accurately than fluorescence bronchoscopy alone, showing a sensitivity and a specificity of 71.4% and 90.9%, respectively (72). Other studies showed that HMB combined with NBI was capable of detecting the onset of angiogenesis in the multi-step carcinogenesis of the lung, with or without using fluorescence techniques (70-72).
The natural history of bronchial intraepithelial neoplasia
The natural history of preinvasive lesions and CIS has been evaluated in several relatively small longitudinal studies. In general, the rate of progression of CIS to invasive SqCC was found to range from 39% to 69%, depending on the patient population and length of follow-up (24,50,75). Other studies evaluated rates of progression to a higher grade or CIS among the different grades of dysplastic preinvasive lesions. In one study, rates of progression to CIS were 14% and 28% for lesions showing moderate or severe dysplasia, respectively (76). In another study, 32% of lesions showing severe dysplasia and 9% of lesions with mild/moderate dysplasia progressed to CIS or SqCC when followed for a range of 11 to 21 months whereas regression was observed in 54% of the preinvasive lesions (19,77).
Several factors complicate the evaluation of the natural history of preinvasive airway lesions, first of all the difficult in differentiating the various degrees of dysplasia or distinguishing severe dysplasia versus CIS (45). Furthermore, there was considerable heterogeneity across studies in inclusion and exclusion criteria, frequency of surveillance bronchoscopies, length of follow-up, study end points, and the definition of progression (45).
The American College of Chest Physicians (ACCP) guidelines proposed the following indications for WLB and/or AFB (45): in patients with severe dysplasia or CIS in sputum cytology who have chest imaging studies showing no localizing abnormality, the use of WLB is suggested, with AFB which may be used as an adjunct when available. Patients with known severe dysplasia or CIS of central airways should be followed with WLB or AFB, when available. WLB or AFB is also suggested for patients with early lung cancer who will undergo resection for delineation of tumor margins and assessment of synchronous lesions. For patients being considered for curative endobronchial therapy to treat CIS or early central lung cancer, WLB is suggested over routine use of AFB. Finally, for patients with superficial limited mucosal lung cancer in the central airway who are not candidates for surgical resection, endobronchial treatment with PDT, brachytherapy, cryotherapy, or electrocautery is recommended.
Technical and operational aspects of endoscopic tracheobronchial dis-obstruction
Endoscopy suite: setting, staff and organization
Setting up and running an endoscopy unit is a complex topic, of particular interest to directors and nurse managers, with an expanding literature. Some basic principles are mentioned here briefly. A bronchoscopy unit consists of a dedicated location where flexible or rigid procedures are regularly performed, with available equipment necessary to provide bronchoscopy and related interventional procedures, and a team composed of well-trained bronchoscopists, anesthesiologists and nurses/surgical technicians. Many components have to be taken into account when a conceiving a modern endoscopic suite and those are strictly related to logistic, technologic, scientific, technical, safety, educational, and communicational issues. From the patient’s perspective, endoscopy consists of reception, preparation, procedure, recovery and discharge. Enabling these activities are a series of other functions, which include scheduling, cleaning, preparation, maintenance and storage of equipment, reporting and archiving, and staff management.
The ideal bronchoscopy unit should therefore include adequate space for the storage of bronchoscopic equipment, a waiting/recovery area for preparing and assisting the patient after the procedure, equipped with an oxygen supply, vacuum suction, and adequate monitoring systems (BP, HR, oxygen saturation, EKG, defibrillator for resuscitation including the emergency intubation’s kit and chest tube insertion), a dedicated suite to perform the procedures (78). Moreover, a separated area devoted to instrument’s processing and sterilization is strictly required.
The suite should be accessible for a stretcher or bed, and the examination table should be accessible from all sides.
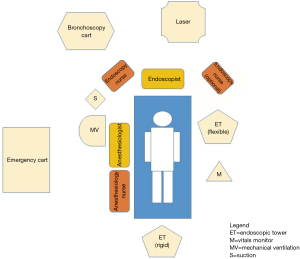
Bronchoscopy unit should satisfy with precise ergonomic principles (see Figure 8). The endoscopic tower for flexible bronchoscopies, for example, should be located on the left of the endoscopist to allow the instrument’s light guide and connectors to be optimally placed; alternatively, if a rigid scope is used, it can be located at the end of the operating table. Consequently, the assistant nurse should station on the bronchoscopist’s right side to have a direct access to the bronchoscopic cart and to provide the operator with every needed device (forceps, saline, TBNA, laser probe, etc.). Finally, the anesthesiologist, should be placed on the patient’s right side, in order to act without interfering with the operating theater and be able to monitor vital’s signs.
Every team member, composed by a bronchoscopist, an anaesthesiologist and related nursing staff should have received appropriate training certification before to be admitted to perform interventional pulmonology procedures.
Training in rigid bronchoscopy should be reserved to physicians who have had previously extensive experience with flexible bronchoscopy and endotracheal intubation (20). Trainees should perform at least 20 supervised rigid bronchoscopy procedures before attempting this procedure alone. To maintain competency, the procedure should be performed at least 10–15 times/years. (79)
The anaesthesiologist should be proficient with conventional, jet, and single-lung ventilation.
For interventional procedures, at least one to two nursing assistants are needed. General anaesthesia is administered intravenously. Ventilation techniques that can be used include: spontaneous ventilation, spontaneous assisted ventilation, controlled ventilation with Venturi Jet, high-frequency (HF) ventilation, or closed-circuit positive pressure ventilation.
Set of instruments
Several types of both flexible and rigid bronchoscopes will be required if interventional endoscopic procedures are performed.
Rigid bronchoscopy may require a larger number of equipment (Figure 9):
Classic ventilating trachea-bronchoscopes: open tubes with bevelled distal ends, side ports to maintain bilateral ventilation in bronchoscopes for distal airway endoscopy, a proximal end that contains several ports for light guides, telescopes, introduction of instruments, and assisted ventilation.
Flexible versus rigid bronchoscopy
Rigid bronchoscopy has a history of more than 100 years. In the late 19th century and early 20th the development in technology of surgical instruments brought to production of affordable and effective metal devices. Metallic tubes shaped to penetrate without damage the internal cavities of the human body were designed and experimented. The problem of bringing the light inside of the human cavities was solved with mirrors first, and later with electric miniaturized bulbs. When the technology of glass fibers was employed finally, a cold and powerful light supply was available. The production of wonderful optic crystal and of miniaturized telescopes allowed in the second half of 20th century the production of effective rigid bronchoscope able to explore the main airways to recover samples of tissue, blood, phlegm, to remove foreign bodies and endoluminal tumors.
In late 70s of 20th century the technology of optic fibers was employed to produce endoscopic instruments that were actually able to transmit the images and the light along curved fibers. The new bronchoscopes revealed to be a real revolution in the exploration of the airways, allowing to reach peripheral bronchi and also areas difficult to explore with the rigid optics, as upper lobes.
This seemed to be the ending of the era of rigid instruments. The new flexible bronchoscopes were easier to use (and to learn), more comfortable to the patients, able to consent new procedures, like the bronchoalveolar lavage, the transbronchial biopsy, the needle puncture of lymph nodes. In rich countries, like US and Japan, in whom the access to new technologies was simpler, the use of rigid bronchoscopes was almost abandoned.
Otherwise the same technology was the cause of a renewed interest in rigid instruments, when laser beam entered in medical practice. The resistance to the heat and the presence of a huge metal working channel, if compared to the small 2 mm plastic channels of bronchoscopes made these instruments more attractive for laser treatments inside of tracheobronchial tree. The removal of large tumors, blood clots, the possibility to use large forceps, to ventilate a patient under general anesthesia, was clearly favoring rigid instruments.
Furthermore, before the electronics revolution of video cameras, the vision quality of rigid telescopes was enormously better than the reticulated, dark field of view of fiberoptic bronchoscopes. When endobronchial stents or prostheses were proposed and developed in order to maintain the patency of the airways, due to the difficulties in management of stenting procedures by means of flexible instruments, rigid bronchoscopes became the gold standard in laser and operative bronchoscopy.
The new flexible HR video bronchoscopes, whose quality, in terms of vision and of resistance, has enormously increased in the last years, are nevertheless less effective than rigid ones.
Various angled telescopes that can be connected to video cameras and provide excellent airway visualization and documentation.
The main features of rigid and flexible bronchoscopes are listed in Table 1.
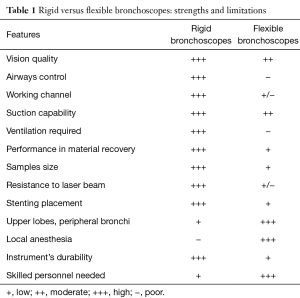
Full table
Dedicated instruments for operative endoscopy include, basically, giant forceps for large size biopsies and foreign body/tumor removal, and large suction channels for aspirating secretions or blood.
Further devices required in interventional pulmonology (see also Table 2) are as below.
Full table
Lasers
“Laser” is an acronym for light amplification by stimulated emission of radiation. A laser is created when the electrons in atoms in special glasses, crystals, or gases absorb energy from an electrical current or another laser and become “excited.” The excited electrons move from a lower-energy orbit to a higher-energy orbit around the atom’s nucleus. When they return to their normal or “ground” state, the electrons emit photons (particles of light).
Only lasers with wavelengths that can be delivered through an optical fibre are suitable for laser bronchoscopy (80). These include the potassium titanyl phosphate (KTP), argon dye, yttrium aluminium perovskite (YAP), Nd:YAG and diode lasers (81). The Nd:YAG laser is currently preferred for airway resection because of its predictable effects on living tissue (i.e., photocoagulation or vaporization), depending on the amount of energy applied (82) (See Figure 10).

Endobronchial electrosurgery (EES) and APC
The use of electrocautery through the bronchoscope was first reported in the early 1980s with varying degrees of success (84). Despite its low cost, EES failed to gain popularity due to cumbersome delivery systems and complications associated with the power units. Since the evolution of a new generation of electrosurgical devices, there has been renewed interest in its application in the endobronchial tree (85). EES (synonyms: electrocautery or diathermy) is defined as the application of a HF electrical current via a probe to coagulate or vaporize tissue (84,86). APC is a noncontact mode of mono-polar electrical coagulation; argon gas is used as the conductive medium (87).
Endobronchial cryotherapy
Cryotherapy is the therapeutic application of extreme cold for local destruction of living tissue. In contrast to laser or electrosurgery, cryodestruction is delayed, occurring from a few hours to a few days after application (88). Cryotherapy can be performed either through a rigid or flexible bronchoscope, requiring a rigid or flexible cryoprobe. Commonly used cryogens are nitrous oxide (N2O) and liquid nitrogen (N2) (89). Bronchoscopic cryotherapy can be used in a variety of clinical scenarios, including the treatment of malignant and benign central airway obstruction and low-grade airway malignancy, foreign body removal or cryoextraction, endobronchial biopsy, and transbronchial biopsy. Unfortunately, the bulk of the experience with bronchoscopic cryotherapy consists of uncontrolled case series of malignant central airway obstruction, so further investigation is currently needed (90) before this approach may become suitable for routine clinical applications.
Airway stents
Airway stents or tracheobronchial endoprostheses are devices used to re-establish airway patency, either to support the tracheobronchial wall in stenosis or malacia or to seal off airway fistulas. Most stents are available in various shapes (straight, Y-shaped, J-shaped), diameters, and lengths. Stents are made of polymers, metal, or a combination of both (hybrids) (91). Delivery devices include a rigid bronchoscope and its accessories, specially-designed rigid stent introducer systems (generally for polymer and silicon-made stents), a flexible bronchoscope, and its accessories, and specially designed flexible stent introducer systems (generally for metal stents and hybrids) in certain situations (i.e., narrow, curvilinear stenosis) (92). Stent insertion should be reserved to bronchoscopists with extensive experience in rigid/flexible bronchoscopy and endotracheal intubation.
The silicon stent insertion technique can be performed exclusively by rigid bronchoscopy and requires dedicated charging and delivery devices. Briefly, these stents are placed inside a delivery tube of the appropriate caliber based on the size of the rigid bronchoscope used for intubation and a plunger system is used to push it out of the introducer. Fine adjustments after stent deployment are performed with grasping forceps (Figure 11) under direct visualization.
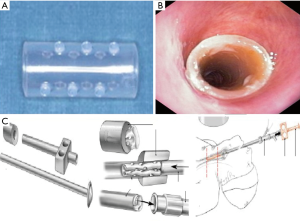
For all these procedures trainees should perform 50 supervised procedures before attempting this procedure alone. In order to maintain competence, 5±10 procedures/years should be performed (79).
Debulking and coring out of locally advanced endobronchial tumors
As already described, about 30% of patients with lung neoplasms will develop an obstruction of main airways along with the progression of the disease (2). The obstruction dramatically influences the respiratory and clinical conditions of the patients, causing cough, dyspnea, obstructive pneumonia. Restoring the patency of the airways improves the oxygenation and the tolerance to effort, and reduces the cough. The early palliation of symptoms is important for the quality of the life, but may also enable the patients to afford surgery, radiotherapy or chemotherapy and, importantly positively affects survival in patients with advanced lung cancer (93). The endoscopic procedures are effective in restoring the patency using different methods, but it should be underlined that an accurate selection of the patients is critical.
The atelectasis of the lung induces a progressive decay of the parenchyma. The lung may still recover and return to a normal function if it is re-ventilated within a time interval varying between 4 and 12 weeks, depending on the entity of the occlusion and on the presence of germs and over-infection in the collapsed parenchyma (94). Furthermore, also the residual vascularization of the lung is critical. The dis-obstruction has no sense if the vascularization is compromised, like in those tumors infiltrating the vessels (95). The presence of a patent bronchus distal to the tumor obstruction is moreover necessary. In patients with mucosal infiltration of the distal tree the occlusion will likely relapse within a short time.
Finally, the involvement of distal carinas and bifurcations generally contraindicate the treatment (96).
From a technical point of view, we have to distinguish occlusions deriving from endobronchial growth tumors, infiltrating tumors, extrinsic compression. In all cases a rigid endoscope has to be employed, in order to remove large fragments of tumor, aspirate significant amounts of blood and of secretions, and/or ventilate the patients without risks of fire (12). A rigid endoscope allows the simultaneous introduction of a telescope, of a large aspiration catheter, of a laser fiber and a large rigid forceps (see Figure 9). If needed, blocking balloons, haemostatic tissue or stents may be also inserted through the rigid scope operating channel.
In endoluminal dis-obstruction, the laser assisted resection is similar to that described for benign endoluminal tumors and requires the same systematic approach.
The laser beam, generated by a Yag, or a Diode or Perovskite laser, is used to devascularize the tumor. If the tumor has a recognizable implant base, we use the fiber, first, to coagulate the peduncle and, later, to dissect it with a contact tip. If this is not possible due to large and irregular implant base, the laser beam is used to coagulate the mass and then a debulking with tip of the rigid bronchoscope by the so-called “coring out” technique is performed (see Figure 10).
The tumor is then removed and the haemostasis is warranted by mechanical compression exerted by the bronchoscope and/or by laser coagulation of infiltrated area.
In narrowing of the airway due to infiltration or compression, a mechanical resection and laser treatment is usually not possible. In these patients, a stent may be used to restore patency. Many kinds of stents have been proposed. The most used for this purpose are silicon stents, which have efficient radial resistance to the compression, are not infiltrated by the tumor, may be easily removed and replaced with a new one if necessary. Their insertions always require a rigid bronchoscope and a skilled endoscopist. Despite silicon stents are usually more effective than metallic self-expandable ones, plugging from secretions is possible and migration has been described. Self-expandable uncovered stents are more simple to insert, but may be infiltrated by the tumor, are almost impossible to remove, and may be compressed (97). Fracture of the metallic wire is also possible and vascular and wall damage has been described (98). Self-expandable, covered stents combine advantages and disadvantages of both, with a simple insertion, resistance to infiltration, but also possibility of migration, of rupture and exertion of granulomas.
Alternative approaches and future perspective for endobronchial treatment of lung cancer
We have discussed in deep how, in the past few decades, a huge effort to achieve a more effective treatment of patients with obstructive endobronchial tumors, has been pursued. However, all of these interventional bronchoscopic methods are mainly designed for acute therapy of the airway occlusion and do not treat the underlying disease. Indeed, although indications for endoscopic treatments were mainly for palliation of advanced endobronchial cancerous lesions, there is increased attention devoted to adjuvant therapy and even cure of early lung cancer.
Endobronchial intra-tumoral chemotherapy (EITC)
Celikoglu et al. have observed and reported that direct injection of chemotherapeutic agents via needle-catheter into endobronchial tumors is remarkably effective for the debulking of airway obstructions and is also an effective treatment modality for inoperable patients (99). In other recent clinical studies, Celikoglu et al. also demonstrated the practical clinical feasibility and effectiveness of intratumoral chemotherapy as a local neoadjuvant therapy in newly diagnosed lung cancer patients before radiotherapy or before resection surgery (100-103).
There are several potential advantages for direct intratumoral drug injection. These include: (I) assured precision in the local delivery of drugs; (II) complete perfusion of drug within and around the lesion; (III) dramatically higher tumor tissue concentrations than is achievable by conventional systemic chemotherapy; and (IV) little or no systemic toxic side effects (104). Intratumoral chemotherapy can uniquely provide very high drug concentrations in the endoluminal, submucosal, intra-mural, and peribronchial areas. Indeed, endobronchial intratumoral injection of anticancer agents presents a particularly attractive strategy for local-regional chemotherapy by enabling full penetration of bulky tumors with drug. This procedure is significantly different from endoscopic thermal procedures which have some efficacy for only endoluminal exophytic tumors, and differs from PDT and brachytherapy which may have some efficacy for only superficial tumor growths.
EIT chemotherapy should not be simply regarded as a technique for ablation of endobronchial tumor bulk like other endoscopic ablative procedures. It is rather a distinct form of chemotherapy in affecting cytotoxic action on a large local tumor cell burden with accompanying tumor cell death and tumor tissue necrosis (105).
Despite this intriguing rationale, however, several issues still need to be clarified before this promising technique may be considered suitable for clinical practice.
Existing studies, in fact, do not yet provide definitive and conclusive data; in particular: (I) no RCT’s designed study have been conducted to confirm data on the efficacy of this method. More importantly, is still not clarified whether this technique might play a role in a neo-adjuvant, adjuvant or palliative setting. (II) No data coming from prospective studies are available on eventually clarify side effects and complication of endoscopic procedure (although no severe risk, included delayed bleeding, have never been reported). (III) The impact on survival has not been assessed in short and long term period. (IV) There is a lack of evidences on the potential role of this technique in the local treatment of other than pulmonary and thoracic cancers (i.e., breast, kidney, haematological, etc.).
EBUS-guided transbronchial needle injection (EBUS-TBNI) for local control of lung cancer
EBUS-guided transbronchial needle aspiration (EBUS-TBNA) has become a standard diagnostic tool and is now a recommended modality for invasive mediastinal staging of non-small cell lung cancer (NSCLC) (106). Further applications of EBUS-TBNA have been developed with the widespread use of the technology. However, there is little experience with the use of EBUS guidance for TBNI (104). Ultrasound guidance provides several advantages. The margins of the lesion can be identified to ensure that the agent is not delivered into normal tissue. Second, the use of ultrasound aided by color Doppler imaging facilitates avoidance of regional vasculature. This mitigates the risk of developing side effects due to systemic distribution of the agent. Third, the curvilinear EBUS image allows for real-time visualization of the agent being delivered. As the injection is performed the image becomes more hypoechoic and tissue swelling is noted. This feature is particularly useful for distributing the agent homogenously throughout the lesion (107).
Bronchoscopy-guided radiofrequency ablation (RFA)
Percutaneous guided-RFA has found clinical applications for lung cancer with good results reported (108). Since the electrode is placed percutaneously directly into the tumor, under cross-sectional imaging guidance such as chest CT, complications such as pneumothorax occur with frequency.
However, it is possible to avoid these complications using the present fibreoptic bronchoscopy guidance. Fiber-optic bronchoscopy-guided cooled-RFA is both safe and technically feasible as the tip of the electrode is confirmed with CT or radiographic imaging guidance. A recent study by Tsushima and colleagues has shown that cooled-RFA had no complications, such as bronchial bleeding or pneumothorax, and can be used with CT or radiographic imaging guidance technologies (109). Again, EBUS can also serve as a guide for endobronchial, paratracheal, peri-bronchial and mediastinal masses RFA. Further research is needed to validate this approach and demonstrate its feasibility, safety and efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cancer Research UK. Lung cancer incidence statistics. Date last accessed: January 3, 2015. Date last updated: May 29, 2014. Available online: www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/incidence/uk-lung-cancer-incidence-statistics
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-97. [Crossref] [PubMed]
- Wilson RW, Kirejezyk W. Pathological and radiological correlation of endobronchial neoplasm. Part I. Benign tumors. Ann Diagn Pathol 1997;1:31-46. [Crossref] [PubMed]
- Scarlata S, Graziano P, Lucantoni G, et al. Endoscopic treatment of primary benign central airway tumors: Results from a large consecutive case series and decision making flow chart to address bronchoscopic excision. Eur J Surg Oncol. 2015;41:1437-42. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Shiraishi T, Yanagisawa J, Higuchi T, et al. Tracheal resection for malignant and benign diseases: surgical results and perioperative considerations. Surg Today 2011;41:490-5. [Crossref] [PubMed]
- Thistlethwaite PA, Renner J, Duhamel D, et al. Surgical management of endobronchial inflammatory myofibroblastic tumors. Ann Thorac Surg 2011;91:367-72. [Crossref] [PubMed]
- Strand J, Maktabi M. The fiberoptic bronchoscope in emergent management of acute lower airway obstruction. Int Anesthesiol Clin 2011;49:15-9. [Crossref] [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1988;94:595-8. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Parr GV, Unger M, Trout RG, et al. One hundred neodymium-YAG laser ablations of obstructing tracheal neoplasms. Ann Thorac Surg 1984;38:374-81. [Crossref] [PubMed]
- Hujala K, Sipilä J, Grenman R. Endotracheal and bronchial laser surgery in the treatment of malign and benign lower airway obstructions. Eur Arch Otorhinolaryngol 2003;260:219-22. [PubMed]
- Bertoletti L, Elleuch R, Kaczmarek D, et al. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest 2006;130:1405-11. [Crossref] [PubMed]
- Nassiri AH, Dutau H, Breen D, et al. A multicenter retrospective study investigating the role of interventional bronchoscopic techniques in the management of endobronchial lipomas. Respiration 2008;75:79-84. [Crossref] [PubMed]
- Sato M, Saito Y, Endo C, et al. The natural history of radiographically occult bronchogenic squamous cell carcinoma: a retrospective study of overdiagnosis bias. Chest 2004;126:108-13. [Crossref] [PubMed]
- Kennedy TC, McWilliams A, Edell E, et al. Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:221S-33S.
- Fujimura S, Sagawa M, Saito Y, et al. A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 2000;89:2445-8. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Hagiwara M, et al. Early hilar lung cancer – risk for multiple lung cancers and clinical outcome. Lung Cancer 2001;33:51-7. [Crossref] [PubMed]
- Breuer RH, Pasic A, Smit EF, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res 2005;11:537-43. [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Sutedja TG, van Boxem AJ, Postmus PE. The curative potential of intraluminal bronchoscopic treatment for early-stage non-small-cell lung cancer. Clin Lung Cancer 2001;2:264-70. [Crossref] [PubMed]
- Moro-Sibilot D, Fievet F, Jeanmart M, et al. Clinical prognostic indicators of high-grade pre-invasive bronchial lesions. Eur Respir J 2004;24:24-9. [Crossref] [PubMed]
- Al-Ayoubi AM, Flores RM. Surgery for lung cancer invading the mediastinum. J Thorac Dis. 2016;8:S889-94. [Crossref] [PubMed]
- Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204-10. [Crossref] [PubMed]
- Neyman K, Sundset A, Naalsund A, et al. Endoscopic treatment of bronchial carcinoids in comparison to surgical resection: a retrospective study. J Bronchology Interv Pulmonol. 2012;19:29-34. [Crossref] [PubMed]
- Dalar L, Ozdemir C, Abul Y, et al. Endobronchial Treatment of Carcinoid Tumors of the Lung. Thorac Cardiovasc Surg. 2016;64:166-71. [Crossref] [PubMed]
- Kajiwara N, Kakihana M, Usuda J, et al. Interventional management for benign airway tumors in relation to location, size, character and morphology. J Thorac Dis. 2011;3:221-30. [PubMed]
- Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934-44. [Crossref] [PubMed]
- Aubry MC, Thomas CF Jr, Jett JR, et al. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: analysis of 28 patients. Chest. 2007;131:1635-43. [Crossref] [PubMed]
- Thomas CF Jr, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest. 2001;119:1143-50. [Crossref] [PubMed]
- Cardillo G, Sera F, Di Martino M, et al. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg. 2004;77:1781-5. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Toninelli C. Curative Bronchoscopic Laser Therapy for Surgically Resectable Tracheobronchial Tumors: Personal Experience. J Bronchol Intervent Pulmonol 2002;9:90-5.
35. Ginsberg RJ, Vokes EE, Ruben A. Non-small cell lung cancer. In: DeVita VT, Hellman S, Rosenberg SA. editors. Cancer: principles and practice of oncology. 5th ed. Philadelphia: Lippincott-Raven, 1997:858-911. - Boyd M, Rubio E. The utility of interventional pulmonary procedures in liberating patients with malignancy associated central airway obstruction from mechanical ventilation. Lung 2012;190:471-6. [Crossref] [PubMed]
- Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. [Crossref] [PubMed]
- Guibert N, Mazieres J, Lepage B, et al. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253-9. [Crossref] [PubMed]
- Hespanhol V, Magalhaes A, Marques A. Neoplastic severe central airways obstruction, interventional bronchoscopy: A decision-making analysis. J. Thorac. Cardiovasc. Surg. 2013;145:926-32. [Crossref] [PubMed]
- Sherani K, Vakil A, Dodhia C FA. Malignant tracheal tumors: a review of current diagnostic and management strategies. Curr. Opin. Pulm. Med. 2015;21:322-6. [Crossref] [PubMed]
- Luo M, Duan C, Qiu J, et al. Diagnostic Value of Multidetector CT and Its Multiplanar Reformation, Volume Rendering and Virtual Bronchoscopy Postprocessing Techniques for Primary Trachea and Main Bronchus Tumors. PLoS One 2015;10:e0137329. [Crossref] [PubMed]
- Goudra BG, Singh PM, Borle A, et al. Anesthesia for Advanced Bronchoscopic Procedures: State-of-the-Art Review. Lung 2015;193:453-65. [Crossref] [PubMed]
- Lorx A, Valko L, Penzes I. Anaesthesia for interventional bronchoscopy. Eur Respir Mon 2010;48:18-32.
- Oviatt PL, Stather DR, Michaud G, et al. Exercise Capacity, Lung Function, and Quality of Life After Interventional Bronchoscopy. J Thorac Oncol 2011;6:38-42. [Crossref] [PubMed]
- Wisnivesky JP, Yung RC, Mathur PN, et al. Diagnosis and treatment of bronchial intraepithelial neoplasia and early lung cancer of the central airways: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e263S-77S.
- Sato M, Saito Y, Usuda K, et al. Occult lung cancer beyond bronchoscopic visibility in sputum-cytology positive patients. Lung Cancer 1998;20:17-24. [Crossref] [PubMed]
- Ishizumi T, McWilliams A, MacAulay C, et al. Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev 2010;29:5-14. [Crossref] [PubMed]
- Travis WD, Colby TV, Corrin B. editors. Histological Typing of Lung and Pleural Tumours. WHO International Histological Classification of Tumours. 3rd ed. Berlin: Springer, 1999.
- Siegfried JM. Biology and chemoprevention of lung cancer. Chest 1998;113:40S-5S. [Crossref] [PubMed]
- Bota S, Auliac JB, Paris C, et al. L. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med 2001;164:1688-93. [Crossref] [PubMed]
- He Q, Wang Q, Wu Q, et al. Value of autofluorescence imaging videobronchoscopy in detecting lung cancers and precancerous lesions: a review. Respir Care 2013;58:2150-9. [Crossref] [PubMed]
- Qu J, Macaulay C, Lam S, et al. Optical properties of normal and carcinomatous bronchial tissue. Appl Opt 1994;33:7397-405. [Crossref] [PubMed]
- Edell E, Lam S, Pass H, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: an international, multicenter clinical trial. J Thorac Oncol 2009;4:49-54. [Crossref] [PubMed]
- Chhajed PN, Shibuya K, Hoshino H, et al. A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. Eur Respir J. 2005;25:951-5. [Crossref] [PubMed]
- Lam B, Wong MP, Fung SL, et al. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Eur Respir J 2006;28:915-9. [Crossref] [PubMed]
- Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 2001;93:1385-91. [Crossref] [PubMed]
- Fuso L, Pagliari G, Boniello V, et al. Autofluorescence bronchoscopy to identify pre-cancerous bronchial lesions. Monaldi Arch Chest Dis 2005;63:124-8. [Crossref] [PubMed]
- Andolfi M, Potenza R, Capozzi R, et al. The role of bronchoscopy in the diagnosis of early lung cancer: a review. J Thorac Dis 2016;8:3329-37. [Crossref] [PubMed]
- Chiyo M, Shibuya K, Hoshino H, et al. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 2005;48:307-13. [Crossref] [PubMed]
- Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: a meta-analysis. J Thorac Oncol 2011;6:1336-44. [Crossref] [PubMed]
- Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: a meta-analysis. Lung Cancer 2011;73:183-8. [Crossref] [PubMed]
- Zhang J, Wu J, Yang Y, et al. White light, autofluorescence and narrow-band imaging bronchoscopy for diagnosing airway pre-cancerous and early cancer lesions: a systematic review and meta-analysis. J Thorac Dis 2016;8:3205-16. [Crossref] [PubMed]
- Zaric B, Canak V, Stojanovic G, et al. Autofluorescence videobronchoscopy (AFI) for the assessment of tumor extension in lung cancer. Technol Cancer Res Treat 2009;8:79-84. [Crossref] [PubMed]
- Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 2004;9:568-77. [Crossref] [PubMed]
- Vincent BD, Fraig M, Silvestri GA. A pilot study of narrow-band imaging compared to white light bronchoscopy for evaluation of normal airways and premalignant and malignant airways disease. Chest 2007;131:1794-9. [Crossref] [PubMed]
- Zaric B, Perin B, Stojsic V, et al. Detection of premalignant bronchial lesions can be significantly improved by combination of advanced bronchoscopic imaging techniques. Ann Thorac Med 2013;8:93-8. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060-5. [Crossref] [PubMed]
- Iftikhar IH, Musani AI. Narrow-band imaging bronchoscopy in the detection of premalignant airway lesions: a meta-analysis of diagnostic test accuracy. Ther Adv Respir Dis 2015;9:207-16. [Crossref] [PubMed]
- Zaric B, Perin B, Becker HD, et al. Combination of narrow band imaging (NBI) and autofluorescence imaging (AFI) videobronchoscopy in endoscopic assessment of lung cancer extension. Med Oncol. 2012;29:1638-42. [Crossref] [PubMed]
- Shibuya K, Hoshino H, Chiyo M, et al. High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax 2003;58:989-95. [Crossref] [PubMed]
- Shibuya K, Hoshino H, Chiyo M, et al. Subepithelial vascular patterns in bronchial dysplasias using a high magnification bronchovideoscope. Thorax 2002;57:902-7. [Crossref] [PubMed]
- Shibuya K, Nakajima T, Fujiwara T, et al. Narrow band imaging with high-resolution bronchovideoscopy: a new approach for visualizing angiogenesis in squamous cell carcinoma of the lung. Lung Cancer 2010;69:194-202. [Crossref] [PubMed]
- van der Heijden EH, Hoefsloot W, van Hees HW, et al. High definition bronchoscopy: a randomized exploratory study of diagnostic value compared to standard white light bronchoscopy and autofluorescence bronchoscopy. Respir Res 2015;16:33. [Crossref] [PubMed]
- Zaric B, Perin B, Stojsic V, et al. Relation between vascular patterns visualized by Narrow Band Imaging (NBI) videobronchoscopy and histological type of lung cancer. Med Oncol 2013;30:374. [Crossref] [PubMed]
- Venmans BJ, van Boxem TJ, Smit EF, et al. Outcome of bronchial carcinoma in situ . Chest 2000;117:1572-6. [Crossref] [PubMed]
- Salaün M, Sesboüé R, Moreno-Swirc S, et al. Molecular predictive factors for progression of high-grade preinvasive bronchial lesions. Am J Respir Crit Care Med 2008;177:880-6. [Crossref] [PubMed]
- Alaa M, Shibuya K, Fujiwara T, et al. Risk of lung cancer in patients with preinvasive bronchial lesions followed by autofluorescence bronchoscopy and chest computed tomography. Lung Cancer 2011;72:303-8. [Crossref] [PubMed]
- Prakash UB, Stelck MJ, Kulas MJ. The bronchoscopy suite, equipment, and personnel. In Prakash UB. editor. Bronchoscopy. New York: Raven Press, 1994:43-51.
- Ernst A, Silvestri GA, Johnstone D, et al. ACCP Interventional Chest/Diagnostic Procedures Network Steering Committee. Interventional Pulmonary Procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693-717. [Crossref] [PubMed]
- Colt HG. Laser bronchoscopy. Chest Surg Clin N Am 1996;6:277-91. [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstruction in 2008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Ramser ER, Beamis JF. Laser bronchoscopy. Clin Chest Med 1995;16:415-26. [PubMed]
- Scarlata S, Fuso L, Lucantoni G, et al. Laser assisted endoscopic resection of a benign endobronchial neoplasm. Asvide 2017;4:330. Available online: http://www.asvide.com/articles/1642
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1985;87:712-4. [Crossref] [PubMed]
- Khemasuwan D, Mehta AC, Wang KP. Past, present, and future of endobronchial laser photoresection. J Thorac Dis. 2015;7:S380-8. [PubMed]
- Pedersen U, Kristensen S, Illum P. Palliative resection with high-frequency cutting loop in malignant tracheobronchial diseases. J Bronchol 1994;1:23-5. [Crossref]
- Sutedja T, van Boxem TJ, Schramel FM, et al. Endobronchial electrocautery is an excellent alternative for Nd:YAG laser to treat airway tumors. J Bronchol 1997;4:101-5. [Crossref]
- Maiwand MO. Cryotherapy for advanced carcinoma of the trachea and bronchi. BMJ 1986;293:181-2. [Crossref] [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small lung cancer. Chest 1992;102:1436. [Crossref] [PubMed]
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc. 2016;13:1405-15. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Dasgupta A, Heights C, Dolmatch BL, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary outcome. Chest 1998;114:106-9. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-42. [Crossref] [PubMed]
- Scarlata S, Rossi Bartoli I, Pedone C, et al. Obstructive atelectasis of the lung. Postgrad Med J. 2016;92:365. [Crossref] [PubMed]
- Reardon ES, Schrump DS. Extended resections of non-small cell lung cancers invading the aorta, pulmonary artery, left atrium, or esophagus: can they be justified? Thorac Surg Clin. 2014;24:457-64. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Radical resections for T4 lung cancer. Surg Clin North Am. 2002;82:573-87. [Crossref] [PubMed]
- Tojo T, Iioka S, Kitamura S, et al. Management of malignant tracheobronchial stenosis with metal stents and Dumon stents. Ann Thorac Surg. 1996;61:1074-8. [Crossref] [PubMed]
- Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg. 2011;6:46. [Crossref] [PubMed]
- Celikoglu SI, Celikoglu F, Goldberg EP. Intratumoral cancer chemotherapy through a flexible bronchoscope. J Bronchol 2004;11:260-5. [Crossref]
- Celikoğlu SI, Karayel T, Demirci S, et al. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J. 1997;73:159-62. [Crossref] [PubMed]
- Celikoğlu F, Celikoğlu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol. 2003;55:1441-8. [Crossref] [PubMed]
- Celikoglu F, Celikoglu SI, York AM, et al. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51:225-36. [Crossref] [PubMed]
- Celikoglu SI, Celikoglu F, Goldberg EP. Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small cell lung cancer with polypoid growth causing erroneous impression of advanced disease. Lung Cancer. 2006;54:339-46. [Crossref] [PubMed]
- Seymour CW, Krimsky WS, Sager J, et al. Transbronchial needle injection: a systematic review of a new diagnostic and therapeutic paradigm. Respiration 2006;73:78-89. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Restoration of Patency to Central Airways Occluded by Malignant Endobronchial Tumors Using Intratumoral Injection of Cisplatin. Ann Am Thorac Soc. 2015;12:1345-50. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc. 2015;12:101-4. [Crossref] [PubMed]
- Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850. [Crossref] [PubMed]
- Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J. 2007;29:1193-200. [Crossref] [PubMed]