Unraveling the mystery of dreams
Dreaming has always fascinated philosophers and psychologists. Freud, Jung, and other scientists made numerous speculations about the generation and purpose of dreams. However, even with decades of scientific research, the neural substrates of dreaming remain unclear. Since first described by Aserinsky and Kleitman in 1953 (1), rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep are two clearly distinct stages of sleep. REM sleep is characterized by wake-like brain activity on electroencephalograms (EEG). NREM sleep refers to other sleep stages apart from REM. The dreaming experience (DE) is often similar to perception in the real world, and brain activity during REM sleep is similar to a state of conscious. Since the 1950s, REM sleep has been considered the only sleep stage that can generate dreams (2). Nevertheless, this proposition was refuted by subsequent findings (3). Experimental data suggest that there is no absolute state that is specific to the generation of dreams. REM sleep is neither necessary nor sufficient for dream generation. In an interesting study that was recently published in Nature Neuroscience, Siclari et al. confirmed that dreams can occur during any sleep stage, albeit less often in NREM sleep (4). In their previous review, Nir and Tononi proposed that “It is time we moved beyond sleep stages when trying to link dream consciousness to neuronal events, and focused on more subtle features of brain activity in space and time” (5). This study made substantial efforts to illustrate the neural mechanisms that underlie dreams and provided preliminary evidence of decoding dream content.
According to brain lesion studies by Solms, bilateral medial occipital-temporal lesions lead to the full or partial loss of visual imagery in dreams (6). Patients with unilateral hemispheric lesions in the temporo-parieto-occipital region presented a more frequent loss of dream recall than patients with lesions outside this area (7). In addition to brain lesion studies, the neuroimaging and neurological literature suggests that brain networks that are implicated in memory and emotional and reward processes and parieto-occipital regions may be involved in dream generation. A meta-analysis of positron emission tomography studies reported the lower activation of cognitive control networks and greater activation of limbic and prelimbic brain regions, reflecting a decrease in self-awareness (8) and increase in subjective experiences in dreams (5). However, the brain regions that are identified are not always consistent, and the precise neural underpinnings of dreaming remain elusive. Using a serial awakening method, Siclari and colleagues investigated the neural mechanisms of dreams using a within-state model separately for REM and NREM sleep because of the substantial distinctions between these sleep stages. According to self-reports after awakening, the state before waking can be divided into three categories: DE, experiencing something but cannot remember the content, or not experiencing anything (NE). By first computing the power spectral density at the source level within the 1–4 Hz frequency band, Siclari et al. found that self-reports of DE were associated with a decrease in low-frequency power in the bilateral parieto-occipital region compared with self-reports of NE in both the REM and NREM neurophysiological states. In NREM sleep, an increase in high-frequency power in the same parieto-occipital region was associated with self-reports of DE compared with NE. A subsequent confirmatory study revealed that the combination of lower low-frequency power and higher high-frequency power in the parieto-occipital region predicted the occurrence of dreams at a high rate of accuracy (87%). These results suggest that the parieto-occipital region, which is related to sensory and visuospatial imagery during wakefulness (9), may be a critical brain region for dream generation.
After identifying the common brain substrates that are associated with dreams, Siclari and colleagues sought to determine the specificities of particular dreams. As an initial step of decoding dream content, they used coarse properties of individual dreams by dividing dream content into different dimensions: thinking and perceiving, face, spatial setting, movement, and speech. The neural mechanisms that underlie these properties have been consistently verified by prior neuroimaging studies in the waking state. They found that specific contents of a subject’s dream were associated with an increase in high-frequency EEG activity in specific cortical areas. These same areas were activated/engaged by the perception of the same content in the waking state (Figure 1). Although large differences are seen between reality, imagination, and dreaming, the “perception” of specific information, regardless of the state of consciousness, activates specific brain regions (10). For example, face processing is always related to the activation of the fusiform face area, regardless of whether the face is real, imaginary, or perceived in dreams. A previous study found that dream movements elicited activation of the sensorimotor cortex (11). A recent study used a combination of machine learning-based pattern recognition analysis and neuroimaging and found that neural representations of visual content in dreams mostly overlapped with actual brain reactivation associated with perception in reality (12). The study by Siclari and colleagues provides remarkable preliminary evidence of deciphering dream content.
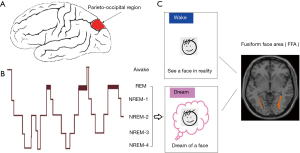
Nonetheless, the study by Siclari et al. raises a few considerations for future studies. First, still unclear are the neural mechanisms that initiate dreams. This study only investigated the preceding period (5 min) of awaking, during which the dream was already in progress, the detected neural activity could only reflect the brain representations for dream content. However, similar to Schrodinger’s cat, there is no direct way to discern whether and when a dream is initiated. Patients with post-traumatic stress disorder (PTSD) repeatedly suffer the same nightmare. Combined with machine learning and the repeated similar dreams of PTSD, a classifier could be trained based on neural substrates to identify when the dream begins. The preceding period would be a start to investigate the initiation of dream. Second, the neural substrates should be interpreted from a network perspective. Changes in functional connectivity between anterior and posterior brain regions may tell a different story about dreams. After training, participants could go to sleep in a scanner, and changes in functional connectivity between the prefrontal and parieto-occipital regions may reveal a biomarker of dreaming. Third, more direct methods could be utilized to verify the hypothesis that similar brain regions are reactivated by the same stimulus during sleep, imagination, and wakefulness. Previous studies have used transcranial direct current stimulation (tDCS) to modulate cerebral activity during sleep and investigate its effects on memory reconsolidation (13). The application of tDCS at 5 Hz increased gamma oscillations during REM sleep (14). tDCS that was applied to the posterior parietal (anode) and frontal (cathode) cortex during stage 2 sleep was consistently related to an increase in the frequency of self-reports of dreams with visual imagery (15). The combination of neuroimaging, machine learning, and brain stimulation techniques may be helpful for elucidating the neural mechanisms that underlie specific dream content. Using neuroimaging to identify the brain representations of specific scenes, neural-based classifiers are trained to estimate the appearance of specific scenes in dreams. If so, then tDCS may be used to directly stimulate brain representations to verify the relationship between dream content and neural substrates. Nevertheless, the findings of Siclari and colleagues make a large step forward in our knowledge of dreams, deepening our understanding of the neural mechanisms that underlie dreams and providing new insights to inspire future studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953;118:273-4. [Crossref] [PubMed]
- Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol 1957;53:339-46. [Crossref] [PubMed]
- Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci 2000;23:843-50. [Crossref] [PubMed]
- Siclari F, Baird B, Perogamvros L, et al. The neural correlates of dreaming. Nat Neurosci 2017;20:872-8. [Crossref] [PubMed]
- Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci 2010;14:88-100. [Crossref] [PubMed]
- Pace-Schott EF, Hobson JA. The neuropsychology of dreams: a clinico-anatomical study. Trends Cogn Sci 1998;2:199-200. [Crossref] [PubMed]
- Murri L, Massetani R, Siciliano G, et al. Dream recall after sleep interruption in brain-injured patients. Sleep 1985;8:356-62. [Crossref] [PubMed]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 2006;50:329-39. [Crossref] [PubMed]
- Busan P, Monti F, Semenic M, et al. Parieto-occipital cortex and planning of reaching movements: a transcranial magnetic stimulation study. Behav Brain Res 2009;201:112-9. [Crossref] [PubMed]
- Fox KC, Nijeboer S, Solomonova E, et al. Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Front Hum Neurosci 2013;7:412. [Crossref] [PubMed]
- Dresler M, Koch SP, Wehrle R, et al. Dreamed movement elicits activation in the sensorimotor cortex. Curr Biol 2011;21:1833-7. [Crossref] [PubMed]
- Horikawa T, Tamaki M, Miyawaki Y, et al. Neural decoding of visual imagery during sleep. Science 2013;340:639-42. [Crossref] [PubMed]
- Marshall L, Mölle M, Hallschmid M, et al. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci 2004;24:9985-92. [Crossref] [PubMed]
- Marshall L, Kirov R, Brade J, et al. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One 2011;6:e16905. [Crossref] [PubMed]
- Jakobson AJ, Fitzgerald PB, Conduit R. Induction of visual dream reports after transcranial direct current stimulation (tDCs) during Stage 2 sleep. J Sleep Res 2012;21:369-79. [Crossref] [PubMed]


