Chemotherapy advances in small-cell lung cancer
Introduction
For several decades, lung cancer has been and remains by far the most common malignancy in the world with an estimated 1.6 million new cases per annum (12.7% of total) (1). It is also the leading cause of cancer-related mortality with an estimated 1.38 million deaths per annum (1). Small-cell lung cancer (SCLC) accounts for between 10% to 15% of all lung cancer cases and is closely linked to the intensity and duration of tobacco smoking (2). As such, typical SCLC patients are elderly, current or past heavy smokers with multiple cardiovascular and pulmonary comorbidities that may impede optimal management. SCLC is characterised by its aggressive nature with rapid growth, paraneoplastic endocrinopathies and early metastasis (3). In developed countries, the incidence of SCLC peaked in the 1980s corresponding to peak rates of cigarette smoking 20 years prior, but is now slowly decreasing due to changing smoking patterns (2).
Untreated SCLC is rapidly fatal within two to four months (3,4). Initial management strategies for SCLC included surgery or radiotherapy alone if deemed unresectable (3,5). Ultimately, both modalities proved to be suboptimal with very low long-term survival rates and early relapses, usually with distant metastatic disease. In 1969, chemotherapy with single agent cyclophosphamide doubled survival when compared to best supportive care alone (6). Following that, combination chemotherapy was trialled and shown to be superior to single agents (7,8). Dramatic response rates, including complete responses (CR), brought forward the tantalising promise of a cure in the 1980s. However, whilst SCLC is initially sensitive to chemotherapy and radiotherapy, relapse is almost inevitable and the efficacy of treatment beyond first line dwindles as it becomes increasingly resistant to treatment (9,10).
For many other solid-tumour malignancies, advances in diagnosis and treatment have resulted in improved survival. However for SCLC, the 5-year survival rates have not improved significantly over the last 40 years and have currently plateaued (2,11,12). In Australia, the 5-year survival rate improved only marginally between the years of 1982-1987 and 2000-2007 with males improving from 3% to 5% and females 5% to 8% (12).
Over the last 30 years, phase III trials of chemotherapy for SCLC have yielded only a two month improvement in median survival time (10). Radiotherapy in the form of prophylactic cranial irradiation (PCI) has provided incremental improvements in those achieving a complete or near-complete response with initial chemotherapy (5.4% improvement in 3-year survival rate from 15.3% to 20.7%) (13).
In contrast to non-small cell lung cancer, the advances in tumour genomics, chemotherapy and targeted therapy have been relatively sluggish for SCLC. There has been a distinct paucity of change to chemotherapy regimens beyond those first used in the 1970s and 1980s and currently platinum-etoposide remains the backbone of therapy (14,15). Recent advances in understanding molecular pathways and genomic aberrations involved in SCLC pathogenesis will hopefully translate into novel therapeutic targets to improve outcomes (16,17).
This review commences with a synopsis of the history and evolution of SCLC and its treatment (Table 1), with a focus on chemotherapy. This is followed by a comprehensive overview of the current systemic options for de novo and relapsed disease as well as novel chemotherapeutic agents and regimens on the horizon.
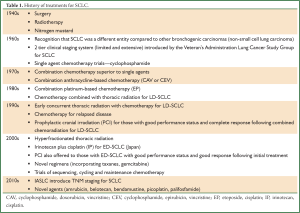
Full Table
SCLC: histology and staging
SCLC was initially believed to be caused by arsenic exposure in miners and was previously labelled as ‘lymphosarcoma of the mediastinum’ (18). In 1926, Barnard discovered that the ‘oat cell sarcoma tumour’ in fact had an epithelial origin arising from the lung (19). In 1967, the World Health Organisation (WHO) first categorised SCLC into four histological subtypes based on Barnard’s observations including: (I) lymphocyte-like; (II) polygonal; (III) fusiform and (IV) other (3,9). Numerous revisions were made by the WHO before the International Association for the Study of Lung Cancer (IASLC) modified it further in 1988, replacing the term ‘oat-cell’ with ‘small cell carcinoma’.
The original staging system for SCLC was introduced in 1968 by the Veterans Administration Lung Cancer Study Group and consisted of two clinical subgroups namely ‘limited disease’ (LD-SCLC) and ‘extensive disease’ (ED-SCLC) (20). LD-SCLC was defined as tumour and nodes confined to one hemithorax and able to be encompassed within a single radiotherapy port, whilst all else was ED-SCLC (11,20).
Approximately 30-40% of patients present with LD-SCLC and are optimally treated with combination chemotherapy with thoracic radiation. Median survival is between 15 to 20 months with 2- and 5- year survival rates of 20-40% and 10-20% respectively (21). Unfortunately, most patients (60-70%) will present with ED-SCLC and are treated with combination chemotherapy resulting in a median survival between 8 to 13 months. Moreover, both 2- and 5-year survival rates remain poor at approximately 5% and 1-2% respectively (21).
As most of SCLC literature utilises the two-subgroup clinical staging system, it remains relevant for clinical decision-making regarding therapy. However there are significant differences between survival outcomes within the ‘limited disease’ subgroup. When LD-SCLC is further stratified according to the IASLC’s Tumour, Node, Metastasis (TNM) classification (7th edition 2010), 5-year survival rates range from 38% for stage IA to 9% for stage IIIB (11). This highlights the need for more precise stratification and as such the TNM staging is now recommended at least in clinical trials for non-metastatic disease (11,15).
Evolution of combination chemotherapy
Although combination chemotherapy is now widely accepted to be integral in the treatment of all stages of SCLC, this contrasts with historical systemic strategies (15,22,23). In the 1940s, initial efforts to treat SCLC involved surgery until radiotherapy was shown to be superior, even for operable cases in 1969 (5,14,18). Alkylating agents such as nitrogen mustard were used as early as 1942, but at the time, the true nature of SCLC was yet to be discovered and all bronchogenic carcinomas were treated similarly (18,23-26). Nitrogen mustard did improve inoperable bronchogenic carcinoma’s median survival time from 93 to 121 days (notably only 81 of 468 had oat cell carcinoma) (25,26). In 1962, Watson and Berg argued that ‘oat cell carcinoma’ with its distinctly aggressive nature and propensity for early metastasis might be better treated with combination intensive chemotherapy and radiation rather than local treatments such as surgery or radiation alone (23).
Cyclophosphamide was the first cytotoxic chemotherapy agent to demonstrate a statistically significant survival advantage over placebo [1969] for bronchogenic carcinoma including SCLC (4.0 vs. 1.5 months) (6). Furthermore, in 1979, the combination of cyclophosphamide-based chemotherapy plus thoracic radiation was shown to be superior compared to radiotherapy alone (7,27).
Following these promising results with cyclophosphamide, further single agent cytotoxics were studied with objective overall response rates (ORR) of up to 62% including; anthracyclines, etoposide, tenoposide, ifosfamide, hexamethylmelamine, cisplatin, carboplatin, vindesine, vincristine and nimustine (28). From this, it was recognised that the epipodophyllotoxins (etoposide and tenoposide) were some of the most active single agents in SCLC (29-32). Indeed, a randomised trial using three different schedules of etoposide showed response rates between 20-62% (33). Alkylating agents including ifosfamide showed response rates of up to 46% (28) and other alkylators including cisplatin and carboplatin were less active but animal studies suggested synergism with etoposide (28-33). As single agents in heavily pre-treated SCLC, cisplatin and carboplatin had ORRs of 15% and 24% respectively (28).
Following this, the combination of cyclophosphamide with an anthracycline (doxorubicin or epirubicin) and vincristine (CAV or CEV) was investigated. In extensive disease, CAV showed 14% CR rate, 57% ORR and median survival of 26 weeks. In limited disease, CAV had a 41% CR rate, 75% ORR and median survival of 52 weeks (8). The addition of etoposide to the CAV regimen (CAVE) did not reproducibly improve survival but came at the cost of increased haematological toxicity (34). Thus until the mid-1980s, CAV was the standard regimen for first line induction chemotherapy (34,35).
In cases where anthracyclines were contraindicated due to severe cardiac or hepatic dysfunction, an alternative regimen was suggested using a combination of the most active and synergistic drugs in pre-clinical models. VP-16 or etoposide was combined with cisplatin (EP) and the combination yielded an impressive ORR of 86-89% (29,30). ORR approximated 55% in those refractory to previous anthracycline-based chemotherapy. Median survival times were 70 and 43 weeks for limited and extensive stage disease respectively (30,31). In the realms of SCLC management, this study proved to be ground-breaking as it yielded responses comparable to anthracycline-based chemotherapy in patients with poorer performance status, serious cardiac disease or extensive liver and brain metastases (30,31).
Following this, direct comparisons between CAV and EP showed equivalent response rates (61% for CAV versus 51% for EP) (36). CR rates and median survival rates were 10% versus 7% and 8.6 versus 8.1 months for CAV and EP respectively (36). Alternating CAV and EP was also investigated and was no different except for a trend towards longer median time to progression (4 months with EP versus 5.2 months with EP/CAV alternating) (36). However, Fukuoka et al. conducted a similar trial in Japan showing that EP or CAV alternating with EP (CAV/EP) had significantly higher response rates compared to CAV (78%, 76% and 55% respectively) (37). Survival times favoured the alternating regimen CAV/EP (11.8 months) compared to EP (9.9 months) (P=0.056) or CAV (9.9 months) (P=0.027) (37).
These results favouring platinum-containing regimens have been confirmed by a subsequent randomised phase III trial with 5 years of follow up (38). In LD-SCLC, EP was superior to CEV with 2- and 5-year survival rates of 25% and 10% respectively in the EP arm compared to 8% and 3% in the CEV arm (P=0.0001) (38). For ED-SCLC, there was a trend towards survival benefit with EP over CEV but these were not statistically significant with median survival 8.4 versus 6.5 months respectively (38). When combined with concurrent thoracic radiation, EP is also better tolerated than anthracycline-based regimens (e.g. less oesophagitis and pneumonitis) and so became the most frequently used chemotherapy regimen for SCLC (10,22,30,31,37-40).
The increasing use of platinum in a host of solid tumours has stimulated a plethora of studies comparing its efficacy with non-platinum regimens along with head to head comparisons between cisplatin and carboplatin. With respect to SCLC, a meta-analysis by Pujol et al. found that cisplatin-based regimens had an increased probability of response over those without cisplatin (OR 1.35, 95% confidence interval of 1.18-1.55) (41). Cisplatin is associated with significant nephrotoxicity, neurotoxicity and gastrointestinal adverse effects whereas carboplatin is associated with more myelosuppression (42). The COCIS meta-analysis by Rossi focused on whether or not cisplatin was required or if carboplatin could be substituted (42). It suggested that carboplatin-based regimens were equivalent in terms of ORR, progression-free survival (PFS) and overall survival (OS) compared to cisplatin-based regimens (42). Thus it seems reasonable to substitute carboplatin for cisplatin to avoid non-haematological toxicities.
First-line chemotherapy
Current combination chemotherapy with either EP or CAV achieves partial or complete responses rates between 50% to 85% alongside median survival times ranging from 9 to 12 months (4,10). In the hope of improving the outlook for SCLC, several novel agents have been investigated upfront in view of encouraging preliminary results witnessed with these drugs in relapsed disease. Much of the progress seems to have been focussed around the DNA topoisomerase enzymes that are critical for DNA replication and ultimately cell survival (Table 2). Dual inhibition of both topoisomerase I and II can produce significant cytotoxic effects by arresting both DNA and RNA replication by maintaining torsional stresses that ultimately impede tumour cell division (53).
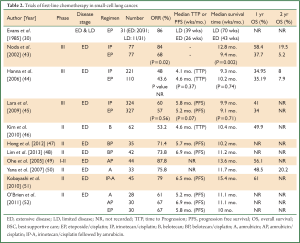
Full Table
Irinotecan
Irinotecan, a topoisomerase I inhibitor, has shown much promise in numerous phase II trials. The Japanese Clinical Oncology Group (JCOG) conducted a phase III trial combining cisplatin with irinotecan (IP) and compared it to EP in treatment naïve ED-SCLC (43). The trial was terminated early due to an interim analysis showing a significant benefit in median survival with IP compared to EP (12.8 versus 9.4 months respectively, P=0.002) (43). OS rates at 2 years were 19.5% and 5.2% respectively suggesting new hope in ED-SCLC (43). Myelosuppression was more common with EP whilst diarrhoea was more common in the IP arm (43).
Whilst this regimen was adopted as first-line therapy for SCLC in Japan, confirmatory studies were required prior to changing standard practice in other countries. Two large North American studies looked at the IP combination but found conflicting results to the JCOG study (44,45). The first used a slightly modified protocol (cisplatin 30 mg/m2 i.v.i. plus irinotecan 65 mg/m2 i.v.i. on days 1 and 8 every 21 days) compared to the JCOG (cisplatin 60 mg/m2 i.v.i. day 1 and irinotecan 60 mg/m2 i.v.i. on days 1, 8, and 15 q28 days) and found no differences in survival (44). The follow up SWOG S0124 trial used an IP protocol identical to that used in the JCOG trial but found that IP was equivalent to, but not superior to EP, both in terms of ORR and OS (45). It is postulated that pharmacogenomic variability amongst different ethnic populations could be a potential reason for the differing results; a concept covered further in this review.
Belotecan
Belotecan is a novel camptothecin derivative that inhibits topoisomerase I and positive results from single agent therapy in previously untreated ED-SCLC were seen in a phase II trial (46). It had an impressive ORR of 53.2%, time to progression (TTP) of 4.6- and 10.4-month median OS (46). The most common toxicity was haematological with up to 71% grade 3/4 neutropenia (46). Subsequently, belotecan was combined with cisplatin in two phase II studies which both showed an ORR ≥70% and median survival time of ≥10 months (47,48). The results of an ongoing phase III trial (COMBAT) are eagerly anticipated as it compares belotecan-cisplatin with the gold standard EP in chemotherapy naïve SCLC (54).
Amrubicin
Amrubicin is a synthetic anthracycline derivative which shares structural features with doxorubicin and also stabilises the topoisomerase II-DNA complex (55). Its active metabolite amrubicinol is believed to preferentially accumulate in tumour cells and is thus associated with reduced toxicity including anthracycline-cardiotoxicity (53,56,57). A phase II study in previously untreated ED-SCLC patients found that single agent amrubicin had an ORR of 75.8%, median survival time (MST) of 11.7 months and 2-year survival rate of 20.2% (50).
Consequently, the introduction of amrubicin in first line platinum doublet therapy has been investigated with response and survival rates comparable to those documented with platinum-etoposide regimens. Ohe et al. conducted a phase I-II study of amrubicin combined with cisplatin in first line ED-SCLC to determine the maximum tolerated and recommended dose of the novel combination consisting of amrubicin 40 mg/m2/day and cisplatin 60 mg/m2/day (49). They reported an impressive ORR of 87.8% (36 of 41 patients) at the recommended dose schedule. The MST was 13.6 months and 1-year survival rate 56.1%, however these outcomes were counteracted by significant grade 3/4 neutropenia (95.1%) (49).
The West Japan Thoracic Oncology Group 0301 trial was a phase II study investigating sequential triplet chemotherapy with IP followed by amrubicin in previously treated ED-SCLC (51). They reported an ORR of 79% with median PFS 6.5 months. Median OS was 15.4 months but this came at the cost of significant myelosuppression with 91% grade 3/4 neutropenia and 15% febrile neutropenia associated with amrubicin (51).
The EORTC 08062 randomised phase II trial compared amrubicin monotherapy (A) or in combination with cisplatin (AP) versus the standard EP regimen in a non-Asian population (52). Independent reviewer ORR was reported as 61%, 67% and 67% for A, AP and EP respectively (52,58). Although amrubicin is associated with significantly more grade ≥3 haematological toxicities, its impressive response rates are generating interest to further investigate its potential use for SCLC (52).
More recently, Noro et al. conducted a phase II study of non-cross resistant chemotherapy by alternating AP with weekly IP for treatment naïve ED-SCLC (59). Whilst this showed an impressive ORR of 85% including 20% CR, significant myelosuppression was evident with 83.3% grade ≥3 neutropenia. However, weekly IP was associated with significantly more diarrhoea. The MST was 359 days (12 months), median PFS 227 days (7.5 months) and one-year OS rate of 40% (59). Hence, the combination of amrubicin-cisplatin (AP) or alternating AP with IP seems to be a very active regimen for SCLC and AP is now being compared to EP in a phase III trial (60).
Maintenance and consolidation therapy
Due to the propensity for SCLC to promptly relapse, maintenance therapy has been a strategy employed to prolong time to recurrence or progression. The Eastern Cooperative Oncology Group (ECOG) conducted a phase III trial of maintenance topotecan (topoisomerase I inhibitor) for patients with stable or responding disease following four cycles of induction cisplatin-etoposide (61). Although PFS was significantly improved, there was no difference in patient-related quality of life or OS between observation and topotecan arms (8.9 versus 9.3 months; P=0.43) (61). Subsequently, a systematic review and meta-analysis by Rossi et al. found that the addition of maintenance chemotherapy, interferons or biological agents only produced a very small and clinically insignificant survival benefit (62).
Second-line chemotherapy
Although initial objective chemotherapeutic responses to first line treatment are generally observed, this is seldom witnessed beyond this setting with a median OS often <6 months from the point of relapse (63). In line with other diseases where platinum agents represent the core of primary gold standard therapy (e.g. gynaecological cancers), the extent of initial response is a reasonably robust predictor of future outcome in the event of tumour progression. However, as the usual definition of true platinum sensitivity (i.e. platinum free interval of ≥12 months) used in such diseases is rarely applicable in SCLC, historical classifications have adopted a relatively sombre tone reflecting the unrelenting course of this disease.
Initial reports in the 1980s defined chemoresistant patients with disease that had either progressed during first-line therapy or within 90 days of its completion (64). In turn, PFS extensions beyond this time period are generally categorised as having ‘sensitive’ disease. Moreover, in particular cases with both high responses from initial induction chemotherapy and prolonged treatment free intervals (TFI) of >6 months, rechallenging with the same drugs used in primary therapy can achieve response rates of 50% (65,66). These early studies helped define the current nomenclature of ‘sensitive relapsed’ (PFS >3 months), ‘resistant’ (PFS <3 months) and ‘refractory’ (progression through first line treatment) SCLC (67). However, amongst the literature, the ‘refractory’ and ‘resistant’ definitions increasingly appear to be used interchangeably.
With respect to the second-line cytotoxic strategies employed, there is no general consensus on the most effective regimen. However, there is a leaning towards standard therapy with the camptothecin; topotecan, which to date represents the sole agent with FDA approval specifically for this setting. In comparison with commonly used combinatorial approaches such as CAV, topotecan appears to have equivalent response (24.3% vs. 18.3%; P=0.285) and median survival rates (TTP: 13.3 vs. 12.3 weeks; P=0.552; OS: 25 vs. 24.7 weeks; P=0.795) but superior palliation of symptoms such as dyspnoea, anorexia, hoarseness, and fatigue (68). Furthermore, the addition of oral topotecan to best supportive care (BSC) resulted in improved symptom control and significant OS advantages over BSC alone (25.9 versus 13.9 weeks; P=0.01) (69). Of interest, the direct comparisons of oral and intravenous administration have revealed equivalence in terms of response rates (18.3% vs. 21.9%), median OS (33 vs. 35 weeks) and quality of life (70).
Patients with relapsed SCLC will exhibit reasonable responses to other single agents including paclitaxel (71), irinotecan (72), gemcitabine (73) and vinorelbine (74). Nevertheless, although the response rates with such monotherapies are often inferior to combinations of these drugs with platinum agents (75-77), the benefits of combinatorial approaches are often offset by increased toxicity. However, for patients deemed to have sensitive relapse with a PFS of greater >3 months, rechallenging with platinum-based doublets presents a possible option. This approach has been confirmed in a recent meta-analysis conducted by Garassino et al. amongst 161 patients with SCLC undergoing second line therapy having failed EP (78). In this study, subjects were treated independent of their platinum sensitivity and only 30 (18.6%) were rechallenged with platinum. Notably, patients from this particular cohort with platinum sensitive disease showed a trend towards superior ORR (34.5% vs. 17.5%, P=0.06) and OS (9.2 vs. 5.8 months, P=0.08) in comparison with those treated with non-platinum agents (78). Interestingly, clinical benefit (i.e. SD + PR) was obtained in 30% of patients with refractory/resistant disease who underwent platinum rechallenge (78). Despite these results, rechallenging with platinum is mainly reserved for patients with both sensitive relapsed disease and a TFI >6 months.
Despite the modicum of success with such regimens, a clear therapeutic ceiling has been reached with the current armament of cytotoxic agents available for second line treatment and beyond. For this reason, research has focused on developing novel formulations of drug classes such as platinum salts, anthracyclines, camptothecins and alkylating agents; all of which have been the cornerstone of progressive SCLC treatment for several decades (Table 3).
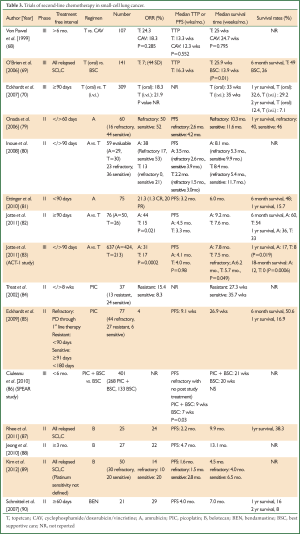
Full Table
Amrubicin
The encouraging results emanating from the aforementioned first-line phase II/III studies with amrubicin containing regimens have stimulated significant interest in relapsed SCLC. Within this sphere, several small Phase II trials have been conducted for both sensitive and refractory SCLC (53) (Table 3) which could potentially help establish an alternative 2nd line regimen to topetecan.
The first of these studies highlighting the salvage potential of amrubicin was published by Onoda et al. in 2006 (79). This multicentre phase II study enrolled 60 patients with relapsed SCLC; 16 refractory (i.e. TTP <60 days from treatment discontinuation) and 44 with sensitive disease (i.e. demonstrable 1st line treatment response and PD >60 days post treatment discontinuation). In line with the current recommended dosing schedule, single agent amrubicin was administered at 40 mg/m2 d1-3 every 3 weeks. The median number of treatment cycles was 4 cycles(range, 1-8 cycles). Interestingly, the ORR for refractory and sensitive patients were almost equivalent at 50% (95% CI, 25% to 75%) and 52% (95% CI, 37% to 68%) respectively. However, superior PFS (2.6 vs. 4.2 months), OS (10.3 vs. 11.6 months) and 1-year survival (40% vs. 46%) favoured patients with sensitive disease (79). With respect to toxicity, grade 3/4 myelosuppression was most commonplace with high rates of neutropenia (83%), followed by anaemia (33%) and thrombocytopenia (20%). Importantly, only 3 patients (5%) experienced febrile neutropenia and no treatment-related deaths were documented (79).
Naturally, these findings fuelled the development of a subsequent study directly comparing the efficacy of amrubicin (40 mg/m2 d1-3 q3 weeks) and topetecan (1 mg/m2 d1-5 q3 weeks) within the second line setting. Another phase II Japanese study conducted by Inoue et al. enrolled 60 SCLC patients pre-treated with platinum-based chemotherapy (80). Of the 59 evaluable, 23 had refractory (defined as no response to 1st line therapy or relapse <90 days of discontinuation) and 36 had sensitive disease. Clear benefits of amrubicin (n=29) over topotecan (n=30) were evident with ORR of 38% (95% CI, 20% to 56%) and 13% (95% CI, 1% to 25%) respectively. In addition, these advantages were highlighted further with patients stratified according to sensitive (53% vs. 21%) or refractory (17% vs. 0%) disease (80). Although the superiority of amrubicin over topotecan reflected in the PFS (3.5 vs. 2.2 months), this did not extend to the MST (8.1 vs. 8.4 months). Rates of neutropenia (79% vs. 43%), febrile neutropenia (14% vs. 3%) and non-haematological toxicities grade >3 were higher in the amrubicin arm and unfortunately, one treatment related death secondary to neutropenia was observed in this group of patients (80).
Analogous to other success stories with novel therapeutics initially trialled in Asian populations [e.g. IPASS in non-small cell lung cancer (NSCLC) (91)], these results were greeted with initial caution as certain pharmacogenomic profiles exclusive to such cohorts could possibly preclude the same responses in Caucasian patients. Specifically, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is an enzyme critically involved in the metabolism of amrubicin and the polymorphisms of this enzyme which are recognised in Asian populations could potentially influence response (92). Consequently, two studies focusing on 2nd line amrubicin treatment in refractory and sensitive SCLC have been conducted in patients from Western populations. With respect to platinum-refractory disease, Ettinger et al. conducted a phase II study with single agent amrubicin in 75 patients who achieved a median PFS of 38 days following 1st line chemotherapy (81). Of these, 69 patients received a median of 4 cycles (range, 1-12 cycles), with a modest ORR of 21.3% (95% CI, 12.7% to 32.3%). In addition 1 CR (1.3%) and 15 PR (20%) were witnessed alongside a PFS and OS of 3.2 months (95% CI, 2.4 to 4.0 months) and 6.0 months (95% CI, 4.8 to 7.1 months), respectively (81). Interestingly, amongst the 43 (57%) patients who failed to respond to initial platinum-based therapy, a 16.3% ORR (95% CI, 6.8% to 30.7%) was observed (81).
The subsequent Jotte et al. study with amrubicin in platinum-sensitive SCLC (i.e. TFI ≥90 days) bore similarities to the Inoue trial by employing a topotecan-containing comparator arm (82). Patients (n=76) were randomised 2:1 to amrubicin (n=50; 40 mg/m2 i.v.i. d1-3, q21 days) or topotecan (n=26; 1.5 mg/m2 i.v.i. d1-5, q21 days). Again, significantly higher ORR was witnessed with amrubicin compared with topotecan (44% vs. 15%; P=0.021) and this also translated into superior median PFS (4.5 vs. 3.3 months) and OS (9.2 vs. 7.6 months). In contrast to the Inoue study, there was a trend towards more myelosuppression (≥ grade 3) with topotecan as opposed to amrubicin (82). In conclusion, the favourable results witnessed with amrubicin in ORR, PFS, OS in sensitive/refractory SCLC and the superiority over topotecan in Asian cohorts are also apparent in patients from the Western world and has consequently stimulated the development of a further larger scale study. Namely, the randomised phase III ACT-1 study aimed to compare the efficacy of 2nd line amrubicin with topotecan in patients with relapsed SCLC (83). In this trial 637 patients were randomized 2:1 to amrubicin (n=424) 40 mg/m2 i.v.i. d1-3 or topotecan (n=213) 1.5 mg/m2 i.v.i. d1-5. In line with similar aforementioned studies with these regimens, the results presented at the 2011 American Society of Clinical Oncology (ASCO) Annual Meeting confirmed that amrubicin had significantly improved ORR compared to topotecan (31% vs. 17%; P=0.0002) (83). Furthermore, despite no differences in PFS, OS trends favoured amrubicin (HR 0.88; 95% CI, 0.73-1.06; P=0.17), with a particular leaning towards patients with refractory disease (HR 0.77; 95% CI, 0.59-1.00; P=0.049) (83).
In addition, small Phase I/II studies have explored the efficacy of combining amrubicin and topotecan as a potential 2nd line regimen (93,94). However despite the 60-70% ORR achieved, any optimism generated from these trials is tempered by unacceptable toxicities including grade 4 myelosuppression, fatal diarrhoea and pneumonitis (94). Nevertheless, the results from the larger amrubicin monotherapy studies have certainly shed significant light on a plausible alternative therapeutic agent that could salvage patients with relapsed SCLC.
Picoplatin
Picoplatin (ZD0473) is a novel organic platinum analogue developed specifically to circumvent the development of platinum resistance mediated by sulphur-containing compounds such as glutathione and metallothionein (95,96); thiol agents that detoxify through avid platinum binding (97). This property extends its anti-neoplastic activity beyond the standard functionality of platinum revolving around DNA alkylation, inter- and intra-strand cross-linking which all facilitate apoptosis. More specifically, an in vitro study has confirmed the reversal of resistance to both cisplatin and carboplatin with picoplatin in platinum resistant H69 and SBC-3 SCLC lines (98). Moreover, it appears that the mechanism of action underlying this phenomenon relates to a decrease in platinum accumulation (98). The first clinical reports confirming single agent activity of picoplatin in relapsed SCLC were published by Treat et al. with a phase II study in SCLC patients with platinum resistant (defined as PD <8 weeks from 1st line platinum based treatment, n=13) or sensitive disease (n=24) (84). The ORR was modest at 15.4% and 8.3% and median OS was 27.3 and 35.7 weeks for the resistant and sensitive groups respectively (84,96). A subsequent larger study with picoplatin monotherapy (150 mg/m2 i.v.i. q3 wks consisted of 77 patients with relapsed SCLC); 57% (n=44) with platinum refractory disease (no response to platinum based therapy), 35% (n=27) with platinum resistance (i.e. relapse <90 days from completing 1st line platinum based therapy) and 8% (n=6) with platinum sensitive disease (i.e. relapse ≥91 days <180 days following completion of 1st line platinum-based therapy) (85). In view of the preponderance of refractory/resistant patients in this study, the ORR was low at 4% with a median PFS and OS of 9.1 and 26.9 weeks respectively (85). With respect to adverse events, the most common grade 3/4 toxicities were thrombocytopenia (48%), followed by neutropenia (25%), and anaemia (20%). Significantly, there were no episodes of febrile neutropenia (85).
Both of these aforementioned studies set the foundations for the Study of Picoplatin Efficacy After Relapse (SPEAR) trial (86). This phase II study consisted of 401 patients with relapsed SCLC (<6 months of completing 1st line platinum-based chemotherapy) randomised 2:1 to picoplatin with BSC (n=268) or BSC alone (n=133). Disappointingly, this trial failed to show any survival advantages in the treatment arm over BSC (P=0.09) (86). However, this may be explained by the unbalanced proportion of patients who received post study chemotherapy in the BSC arm. Interestingly, the subset analysis of refractory patients (i.e. no response or relapse <45 days of completing 1st line platinum-based therapy) who did not receive post-study chemotherapy (n=273), revealed statistically significant PFS advantage favouring the picoplatin arm (P=0.03) amounting to just 2 weeks (86). Despite this, it appears curious why a comparator of BSC was chosen over drugs such as topotecan and amrubicin which both have documented activity in the second line setting. However, with the justifiable nihilism generated by the SPEAR trial amongst lung oncologists, it appears unlikely that such a study will ever be realised.
Belotecan
The modest efficacy witnessed in first-line therapy with the novel topoisomerase I inhibitor; belotecan is also mirrored in a few small studies in the relapse setting. Rhee et al. published the results of a Phase II trial in 25 patients with relapsed SCLC (sensitivity status unknown) treated with belotecan at an initial dose of 0.5 mg/m2 i.v.i. d1-5 q21 days (87). In accordance with toxicity, appropriate dose adjustments were only allowed to be implemented once during subsequent cycles. Out of the 21 evaluable patients, 6 had an objective tumour response; i.e. ORR 24% on the intention to treat analysis. Furthermore, the median PFS and OS were 2.2 and 9.9 months respectively with a 1-year survival rate of 38.3% (87). Although the incidence of grade 3/4 neutropenia was particularly high (88%), severe non-haematological toxicities were not commonplace (87). Similarly, another single agent study was executed in 27 patients with refractory disease who had relapsed within 3 months of obtaining response from platinum-irinotecan based first line therapy (88). The ORR was 22%, with median PFS of 4.7 months (95% CI, 3.6-5.8 months) and a reasonable median OS of 13.1 months (95% CI, 10.4-15.8 months) (88). The latter result is of particular interest as it suggests that belotecan has a role in salvaging patients who are resistant to other topoisomerase I inhibitors.
More recently, Kim et al. have published a larger study investigating the efficacy of belotecan monotherapy in 50 patients with sensitive relapsed (n=20) or refractory SCLC (n=30) (89). The ORR was 14% (95% CI, 4-24%) with a median follow up period of 4.2 months (range, 0.1-19.2 months), and median PFS and OS of 1.6 and 4.5 months respectively. As expected, patients with sensitive relapsed disease fared significantly better compared to refractory counterparts for ORR (20% vs. 10%), OS (6.5 vs. 4.0 months; P=0.003) with a trend towards superior PFS (2.8 vs. 1.5 months; P=0.053). Of note, the multivariate analysis confirmed that the type of relapse and prior response to chemotherapy were independent prognostic factors for OS (89). Again, grade 3/4 myelosuppression was evident with the highest rate associated with neutropenia (54%) followed by thrombocytopenia (38%) and anaemia (32%) (89). Furthermore, one treatment-related death secondary to sepsis was documented in this study. Despite the expected deleterious side effects, belotecan has shown modest activity within the second line setting for both sensitive and refractory SCLC and, as with amrubicin, warrants further exploration in this particular domain.
Future directions and closing remarks
The novel chemotherapeutic agents previously highlighted have indeed provided some optimism, albeit short lived. Other drugs have recently come to the fore and similarly demonstrate variable degrees of efficacy. Bendamustine; a bifunctional alkylating agent, has shown activity in combination with carboplatin in chemotherapy-naïve ED-SCLC. Amongst 55 patients, Koster et al. documented an ORR of 72.7% which included a single complete responder. In addition median TTP (5.2 months), MST (8.3 months) and toxicity profiles all compared favourably in comparison to other standard 1st line platinum containing regimens (99). Bendamustine also appears effective in sensitive relapsed SCLC (i.e. TFI ≥60 days) with ORR 29% and median PFS and OS of 4 and 7 months respectively (90). In view of this preliminary data, a current phase I/IIa study is actively recruiting 30 patients with chemotherapy-naïve SCLC to be treated with 3 cycles of bendamustine combined with irinotecan followed by 3 cycles of standard carboplatin and etoposide (Clincaltrials.gov identifier: NCT00856830).
Following on from the success of pemetrexed in non-squamous non-squamous NSCLC and mesothelioma (100,101), attempts have been made to add this to the armament of therapeutic regimes in SCLC. However the outcomes of two recent phase II studies using pemetrexed monotherapy (500 and 900 mg/m2) in patients with sensitive and refractory relapsed SCLC have been inadequate with minimal efficacy seen in this setting (102,103). These damning results are not entirely unexpected. The discrepancies in the efficacy of pemetrexed in non-squamous and squamous NSCLC seen with the seminal Scagliotti study (100), are based on the higher thymidylate synthase (TS; the principal substrate for pemetrexed) expression associated with squamous histotypes (104). Indeed, a subsequent study has further shown that lower TS expression in advanced non squamous NSCLC is associated with longer PFS (105). Moreover, TS expression in SCLC (both from resected tumours and cell lines) is significantly higher than pulmonary squamous and adenocarcinomas (106,107). Hence, it would appear counterintuitive to adopt strategies involving TS inhibitors for SCLC therapy.
This review has attempted to outline the historical and current progress in the chemotherapeutic management of SCLC. Platinum-etoposide doublets still represent the gold standard of first line therapy and attempts to switch the mode of topoisomerase inhibition may prove to be the most strategic method in improving survival. Although the survival advantages garnered from substituting etoposide for irinotecan in the JCOG study were not recapitulated in the subsequent SWOG S0124 trial; current studies comparing the efficacy of amrubicin or belotecan with platinum with EP (52,54) could potentially change practice. Similarly, both of these agents are showing promise as single agents in salvaging patients with either sensitive or refractory relapsed disease. Taking into consideration the dearth of FDA approved 2nd line regimens in SCLC, there is an obvious urge to develop larger clinical trials with these agents. Furthermore, despite the disheartening outcomes in the SPEAR study, picoplatin may still serve as a viable alternative to either cisplatin or carboplatin in its ability to avert the development of resistance. Hence, trials comparing picoplatin doublets with other platinum containing regimens in previously untreated SCLC could also be considered.
As with other solid tumour types, the successful quest in prolonging survival in SCLC will most likely involve appropriate combinations with the novel drugs outlined in this review alongside emerging therapies such as multi-targeted receptor tyrosine kinase inhibitors or other agents which serve to block signalling cascades inherent to the aggressive tumorigenicity of SCLC (e.g. inhibitors of IGFR, mTOR, MET and hedgehog signalling). Exhaustive preclinical studies with such combinatorial therapies will be required to examine both their efficacy and the inevitable upregulation of resistance pathways that ensue. The development of future clinical trials emanating from these studies will require robust design in order to make significant steps in changing the landscape of this bleak disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [PubMed]
- Kato Y, Ferguson TB, Bennett DE, et al. Oat cell carcinoma of the lung. A review of 138 cases. Cancer 1969;23:517-24. [PubMed]
- Pelayo Alvarez M, Gallego Rubio O, Bonfill Cosp X, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev 2009;CD001990. [PubMed]
- Miller AB, Fox W, Tall R. Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet 1969;2:501-5. [PubMed]
- Green RA, Humphrey E, Close H, et al. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516-25. [PubMed]
- Lowenbraun S, Bartolucci A, Smalley RV, et al. The superiority of combination chemotherapy over single agent chemotherapy in small cell lung carcinoma. Cancer 1979;44:406-13. [PubMed]
- Livingston RB, Moore TN, Heilbrun L, et al. Small-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group study. Ann Intern Med 1978;88:194-9. [PubMed]
- Hann CL, Ettinger DS. The change in pattern and pathology of small cell lung cancer. In: Govindan R. eds. American Society of Clinical Oncology. Alexandria, VA, 2009.
- Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794-801. [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [PubMed]
- Australian Institute of Health and Welfare & Cancer Australia 2011. Lung cancer in Australia: an overview. Cancer series no. 64. Canberra: AIHW, 2011.
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [PubMed]
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973;2:63-5. [PubMed]
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78-98. [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [PubMed]
- Rosell R, Wannesson L. A genetic snapshot of small cell lung cancer. Cancer Discov 2012;2:769-71. [PubMed]
- Haddadin S, Perry MC. History of small-cell lung cancer. Clin Lung Cancer 2011;12:87-93. [PubMed]
- Barnard WG. The nature of the “oat-celled sarcoma” of the mediastinum. J Pathol 1926;29:241-4.
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973;4:31-42. [PubMed]
- Lally BE, Urbanic JJ, Blackstock AW, et al. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist 2007;12:1096-104. [PubMed]
- Johnson DH. Management of small cell lung cancer: current state of the art. Chest 1999;116:525S-530S. [PubMed]
- Watson WL, Berg JW. Oat cell lung cancer. Cancer 1962;15:759-68. [PubMed]
- Levine B, Weisberger AS. The response of various types of bronchogenic carcinoma to nitrogen mustard. Ann Intern Med 1955;42:1089-96. [PubMed]
- Wolf J, Spear P, Yesner R, et al. Nitrogen mustard and the steroid hormones in the treatment of inoperable bronchogenic carcinoma. Am J Med 1960;29:1008-16. [PubMed]
- Wolf J, Yesner R, Patno ME. Evaluation of nitrogen mustard in prolonging life of patients with bronchogenic carcinoma. Cancer Chemother Rep 1962;16:473-5. [PubMed]
- Medical Research Council Lung Cancer Working Party. Radiotherapy alone or with chemotherapy in the treatment of small-cell carcinoma of the lung. Br J Cancer 1979;40:1-10. [PubMed]
- Joss RA, Cavalli F, Goldhirsch A, et al. New drugs in small-cell lung cancer. Cancer Treat Rev 1986;13:157-76. [PubMed]
- Goldhirsch A, Joss R, Cavalli F, et al. Etoposide as single agent and in combination chemotherapy of bronchogenic carcinoma. Cancer Treat Rev 1982;9:85-90. [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471-7. [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. First-line therapy with VP-16 and cisplatin for small-cell lung cancer. Semin Oncol 1986;13:17-23. [PubMed]
- Loehrer PJ Sr, Einhorn LH, Greco FA. Cisplatin plus etoposide in small cell lung cancer. Semin Oncol 1988;15:2-8. [PubMed]
- Cavalli F, Sonntag RW, Jungi F, et al. VP-16-213 monotherapy for remission induction of small cell lung cancer: a randomized trial using three dosage schedules. Cancer Treat Rep 1978;62:473-5. [PubMed]
- Jett JR, Everson L, Therneau TM, et al. Treatment of limited-stage small-cell lung cancer with cyclophosphamide, doxorubicin, and vincristine with or without etoposide: a randomized trial of the North Central Cancer Treatment Group. J Clin Oncol 1990;8:33-8. [PubMed]
- Seifter EJ, Ihde DC. Therapy of small cell lung cancer: a perspective on two decades of clinical research. Semin Oncol 1988;15:278-99. [PubMed]
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [PubMed]
- Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855-61. [PubMed]
- Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665-72. [PubMed]
- Shaw EG, Frytak S, Eagan RT, et al. Etoposide-cisplatin and thoracic radiation therapy salvage of incomplete responders to a noncisplatin induction regimen for limited and extensive small-cell carcinoma of the lung. Am J Clin Oncol 1996;19:154-8. [PubMed]
- Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601-7. [PubMed]
- Pujol JL, Carestia L, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8-15. [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [PubMed]
- Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530-5. [PubMed]
- Kim SJ, Kim JS, Kim SC, et al. A multicenter phase II study of belotecan, new camptothecin analogue, in patients with previously untreated extensive stage disease small cell lung cancer. Lung Cancer 2010;68:446-9. [PubMed]
- Hong J, Jung M, Kim YJ, et al. Phase II study of combined belotecan and cisplatin as first-line chemotherapy in patients with extensive disease of small cell lung cancer. Cancer Chemother Pharmacol 2012;69:215-20. [PubMed]
- Lim S, Cho BC, Jung JY, et al. Phase II study of camtobell inj. (belotecan) in combination with cisplatin in patients with previously untreated, extensive stage small cell lung cancer. Lung Cancer 2013;80:313-8. [PubMed]
- Ohe Y, Negoro S, Matsui K, et al. Phase I-II study of amrubicin and cisplatin in previously untreated patients with extensive-stage small-cell lung cancer. Ann Oncol 2005;16:430-6. [PubMed]
- Yana T, Negoro S, Takada M, et al. Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs 2007;25:253-8. [PubMed]
- Kobayashi M, Matsui K, Iwamoto Y, et al. Phase II study of sequential triplet chemotherapy, irinotecan and cisplatin followed by amrubicin, in patients with extensive-stage small cell lung cancer: West Japan Thoracic Oncology Group Study 0301. J Thorac Oncol 2010;5:1075-80. [PubMed]
- O’Brien ME, Konopa K, Lorigan P, et al. Randomised phase II study of amrubicin as single agent or in combination with cisplatin versus cisplatin etoposide as first-line treatment in patients with extensive stage small cell lung cancer - EORTC 08062. Eur J Cancer 2011;47:2322-30. [PubMed]
- Kimura T, Kudoh S, Hirata K. Review of the management of relapsed small-cell lung cancer with amrubicin hydrochloride. Clin Med Insights Oncol 2011;5:23-34. [PubMed]
- Chonnam National University Hospital. Trial of belotecan/cisplatin in chemotherapy naive small cell lung cancer patient (COMBAT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 July 9]. Available online:
- Hanada M, Mizuno S, Fukushima A, et al. A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res 1998;89:1229-38. [PubMed]
- Metro G, Duranti S, Fischer MJ, et al. Emerging drugs for small cell lung cancer--an update. Expert Opin Emerg Drugs 2012;17:31-6. [PubMed]
- Higashiguchi M, Suzuki H, Hirashima T, et al. Long-term amrubicin chemotherapy for small-cell lung cancer. Anticancer Res 2012;32:1423-7. [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [PubMed]
- Noro R, Yoshimura A, Yamamoto K, et al. Alternating chemotherapy with amrubicin plus cisplatin and weekly administration of irinotecan plus cisplatin for extensive-stage small cell lung cancer. Anticancer Res 2013;33:1117-23. [PubMed]
- Sumitomo Pharmaceutical (Suzhou) Co., Ltd. Phase 3 study of amrubicin with cisplatin versus etoposide-cisplatin for extensive disease small cell lung cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 July 10]. Available online:
- Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593--a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:2114-22. [PubMed]
- Rossi A, Garassino MC, Cinquini M, et al. Maintenance or consolidation therapy in small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer 2010;70:119-28. [PubMed]
- Davies AM, Evans WK, Mackay JA, et al. Treatment of recurrent small cell lung cancer. Hematol Oncol Clin North Am 2004;18:387-416. [PubMed]
- Giaccone G, Donadio M, Bonardi G, et al. Teniposide in the treatment of small-cell lung cancer: the influence of prior chemotherapy. J Clin Oncol 1988;6:1264-70. [PubMed]
- Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409-11. [PubMed]
- Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol 1987;23:1697-9. [PubMed]
- Glisson BS. Recurrent small cell lung cancer: update. Semin Oncol 2003;30:72-8. [PubMed]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [PubMed]
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [PubMed]
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086-92. [PubMed]
- Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347-51. [PubMed]
- Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225-9. [PubMed]
- Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol 2003;21:1550-5. [PubMed]
- Furuse K, Kubota K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Japan Lung Cancer Vinorelbine Study Group. Oncology 1996;53:169-72. [PubMed]
- Goto K, Sekine I, Nishiwaki Y, et al. Multi-institutional phase II trial of irinotecan, cisplatin, and etoposide for sensitive relapsed small-cell lung cancer. Br J Cancer 2004;91:659-65. [PubMed]
- Fujita A, Takabatake H, Tagaki S, et al. Combination of cisplatin, ifosfamide, and irinotecan with rhG-CSF support for the treatment of refractory or relapsed small-cell lung cancer. Oncology 2000;59:105-9. [PubMed]
- Groen HJ, Fokkema E, Biesma B, et al. Paclitaxel and carboplatin in the treatment of small-cell lung cancer patients resistant to cyclophosphamide, doxorubicin, and etoposide: a non-cross-resistant schedule. J Clin Oncol 1999;17:927-32. [PubMed]
- Garassino MC, Torri V, Michetti G, et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer 2011;72:378-83. [PubMed]
- Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 2006;24:5448-53. [PubMed]
- Inoue A, Sugawara S, Yamazaki K, et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol 2008;26:5401-6. [PubMed]
- Ettinger DS, Jotte R, Lorigan P, et al. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol 2010;28:2598-603. [PubMed]
- Jotte R, Conkling P, Reynolds C, et al. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol 2011;29:287-93. [PubMed]
- Von Pawel J, Jotte R, Spigel DR, et al. Randomized phase 3 trial of amrubicin versus topotecan as second-line treatment for small cell lung cancer (SCLC). J Clin Oncol 2011;29:abstr 7000.
- Treat J, Schiller J, Quoix E, et al. ZD0473 treatment in lung cancer: an overview of the clinical trial results. Eur J Cancer 2002;38:S13-8. [PubMed]
- Eckardt JR, Bentsion DL, Lipatov ON, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J Clin Oncol 2009;27:2046-51. [PubMed]
- Ciuleanu T, Samarzjia M, Demidchik Y, et al. Randomized phase III study (SPEAR) of picoplatin plus best supportive care (BSC) or BSC alone in patients (pts) with SCLC refractory or progressive within 6 months after first-line platinum-based chemotherapy. J Clin Oncol 2010;28:abstr 7002.
- Rhee CK, Lee SH, Kim JS, et al. A multicenter phase II study of belotecan, a new camptothecin analogue, as a second-line therapy in patients with small cell lung cancer. Lung Cancer 2011;72:64-7. [PubMed]
- Jeong J, Cho BC, Sohn JH, et al. Belotecan for relapsing small-cell lung cancer patients initially treated with an irinotecan-containing chemotherapy: a phase II trial. Lung Cancer 2010;70:77-81. [PubMed]
- Kim GM, Kim YS, Ae Kang Y, et al. Efficacy and toxicity of belotecan for relapsed or refractory small cell lung cancer patients. J Thorac Oncol 2012;7:731-6. [PubMed]
- Schmittel A, Knödler M, Hortig P, et al. Phase II trial of second-line bendamustine chemotherapy in relapsed small cell lung cancer patients. Lung Cancer 2007;55:109-13. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Ettinger DS. Amrubicin for the treatment of small cell lung cancer: does effectiveness cross the Pacific? J Thorac Oncol 2007;2:160-5. [PubMed]
- Shibayama T, Hotta K, Takigawa N, et al. A phase I and pharmacological study of amrubicin and topotecan in patients of small-cell lung cancer with relapsed or extensive-disease small-cell lung cancer. Lung Cancer 2006;53:189-95. [PubMed]
- Nogami N, Hotta K, Kuyama S, et al. A phase II study of amrubicin and topotecan combination therapy in patients with relapsed or extensive-disease small-cell lung cancer: Okayama Lung Cancer Study Group Trial 0401. Lung Cancer 2011;74:80-4. [PubMed]
- Holford J, Sharp SY, Murrer BA, et al. In vitro circumvention of cisplatin resistance by the novel sterically hindered platinum complex AMD473. Br J Cancer 1998;77:366-73. [PubMed]
- William WN Jr, Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol 2011;8:611-9. [PubMed]
- Mistry P, Kelland LR, Abel G, et al. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer 1991;64:215-20. [PubMed]
- Tang CH, Parham C, Shocron E, et al. Picoplatin overcomes resistance to cell toxicity in small-cell lung cancer cells previously treated with cisplatin and carboplatin. Cancer Chemother Pharmacol 2011;67:1389-400. [PubMed]
- Köster W, Heider A, Niederle N, et al. Phase II trial with carboplatin and bendamustine in patients with extensive stage small-cell lung cancer. J Thorac Oncol 2007;2:312-6. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Socinski MA, Raju RN, Neubauer M, et al. Pemetrexed in relapsed small-cell lung cancer and the impact of shortened vitamin supplementation lead-in time: results of a phase II trial. J Thorac Oncol 2008;3:1308-16. [PubMed]
- Jalal S, Ansari R, Govindan R, et al. Pemetrexed in second line and beyond small cell lung cancer: a Hoosier Oncology Group phase II study. J Thorac Oncol 2009;4:93-6. [PubMed]
- Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589-96. [PubMed]
- Nicolson MC, Fennell DA, Ferry D, et al. Thymidylate Synthase Expression and Outcome of Patients Receiving Pemetrexed for Advanced Nonsquamous Non-Small-Cell Lung Cancer in a Prospective Blinded Assessment Phase II Clinical Trial. J Thorac Oncol 2013;8:930-939. [PubMed]
- Ibe T, Shimizu K, Nakano T, et al. High-grade neuroendocrine carcinoma of the lung shows increased thymidylate synthase expression compared to other histotypes. J Surg Oncol 2010;102:11-7. [PubMed]
- Monica V, Scagliotti GV, Ceppi P, et al. Differential Thymidylate Synthase Expression in Different Variants of Large-Cell Carcinoma of the Lung. Clin Cancer Res 2009;15:7547-7552. [PubMed]


