The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era
The continuing role of chemotherapy for advanced non-small cell lung cancer in the recent targeted therapy era
Despite remarkable advances in the targeted treatment of non-small cell lung cancer (NSCLC) over the past several years, chemotherapy remains of paramount importance in the treatment of advanced NSCLC. Even in patients whose tumors contain EGFR activating mutations or ALK gene rearrangements and are treated with first line tyrosine kinase inhibitors, resistance invariably develops, with chemotherapy remaining the cornerstone of subsequent therapy. In profiling mutations of 1,000 metastatic lung adenocarcinoma patients, although the Lung Cancer Mutation Consortium was able to identify actionable mutations, including molecular aberrations linked to approved drugs and clinical trials in 54% of cases (1), in only a small minority, about 14-18% in Western populations, are there approved targeted drugs (EGFR and ALK TKIs) with which to treat them. As of yet, no drugs targeting oncogenic-driver pathways have been approved in squamous cell lung cancers, though clinical trials are ongoing. With the majority of advanced lung cancer patients not harboring actionable driver mutations with paired targeted agents that effectively improve outcomes, advancing chemotherapy regimens through rational drug combinations and discovery of new potent chemotherapeutics remains critical. This review highlights advances in chemotherapy of advanced NSCLC over the past two years.
Continuing central role of platinum compounds in first line chemotherapy of advanced stage NSCLC
Although recently implemented treatment guidelines recommend that patients with advanced stage NSCLC whose tumors harbor EGFR activating mutations or ALK gene rearrangements be treated first line with erlotinib or crizotinib, respectively, it is with the realization that there is no overall survival benefit to patients with EGFR mutated cancers whether they receive an EGFR TKI first line or second line. This TKI-first recommendation is true even in patients with tumor-related poor performance status (2). For ‘fit’ patients who do not have an oncogene-driven cancer, platinum doublet chemotherapy (with consideration of bevacizumab in non-squamous histology patients) remains the cornerstone of treatment. In an attempt to preserve efficacy and minimize toxicity, platinum-free combinations of newer agents have been tested against conventional platinum-based combinations. Although a recent meta-analysis of 16 randomized trials found that the efficacy was comparable between non-platinum doublets of third-generation agents and platinum-based doublets for pooled overall survival (HR =1.03, 95% CI: 0.98-1.08, P=0.290) (3), all evidence based guidelines support platinum-based therapy as standard of care. Subgroup analyses by different non-platinum doublet protocols revealed that none of the four non-platinum doublets achieved a different survival when compared with platinum-based doublets. The pooled progression-free survival showed that platinum-based doublets may have an advantage over non-platinum doublets (HR =1.06, 95% CI: 1.01-1.12, P=0.03). In this study, a meta-analysis of toxicity could not be performed.
In an attempt to show that platinum compounds were non-essential, a recent Phase III trial in advanced stage NSCLC with performance status 2 randomized patients to receive pemetrexed with or without carboplatin. All efficacy parameters favored the carboplatin-pemetrexed combination over pemetrexed alone: response rate 23.8% vs. 10.3%, PFS 5.8 vs. 2.8 months, and OS 9.3 vs. 5.3 months (4). Clearly, the weight of evidence in all categories of advanced NSCLC without EGFR mutation or ALK fusion favors platinum-based doublet therapy.
Biomarkers to select platinum and non-platinum chemotherapy
Utilizing DNA repair enzymes as biomarkers for better selecting front-line chemotherapy is an area of active investigation. Low ERCC1 expression by either IHC or RT-PCR has been shown in preliminary studies to be a potential biomarker of benefit to platinum compounds and low RRM1 a potential biomarker of benefit to gemcitabine. The ERCC1 enzyme removes platinum-induced DNA adducts, and thus low ERCC1 levels are associated with platinum sensitivity (5). RRM1 is a subunit of ribonucleotide reductase which is the main target of gemcitabine; thus, low RRM1 levels are associated with gemcitabine sensitivity (6). In the recently published phase III TASTE trial in metastatic NSCLC, patients were randomly assigned 2:1 to the experimental arms: (I) gemcitabine/carboplatin if RRM1 and ERCC1 were low; (II) docetaxel/carboplatin if RRM1 was high and ERCC1 was low; (III) gemcitabine/docetaxel if RRM1 was low and ERCC1 was high; and (IV) docetaxel/vinorelbine if both were high (7). Control arm patients received gemcitabine/carboplatin. There were no statistical differences for progression-free survival or overall survival. The authors note they required real-time processing of tumor specimens for ERCC1, RRM1 and in situ protein levels. Therefore day-to-day variations in the reagent assay reliability and processing procedures may have affected the reliability and reproducibility of these assays. A recent attempt to validate ERCC1 by IHC as a prognostic marker to platinum based chemotherapy in the adjuvant setting failed as the same antibody to ERCC1 (but a different batch) could not detect the functional ERCC1 isoform (8).
Thymidylate synthase (TS), the de novo source of thymidylate synthesis, is an essential enzyme for DNA replication and cell growth and one of the primary targets of pemetrexed. Pemetrexed has a potential histology-specific benefit which may be related to higher levels of TS expression in squamous histology of the lung compared to adenocarcinoma with overexpression of TS is related to a reduced sensitivity to pemetrexed (9). In vitro studies have correlated differential expression of TS and pemetrexed sensitivity (10). In an analysis of the largest data set for gene expression of biomarkers reported to date, significant histology-related associations for ERCC1, RRM1, and TS were seen, warranting randomized phase III trials assessing the predictive value of these chemotherapy-related biomarkers (11).
Another biomarker that may assist in chemotherapy selection is SPARC (secreted protein acidic and rich in cysteine), a matricellular glycoprotein that is produced by tumor and/or neighboring stroma. SPARC expression is thought to facilitate the intracellular accumulation of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) (12). Multiple issues in assay development, standardization, tissue processing and antibody reliability have affected the potential utility of these biomarkers to better select rationale chemotherapy combinations in advanced NSCLC. Further development of these predictive biomarkers is of interest to convert chemotherapy into targeted chemotherapy.
Pemetrexed first line therapy for non-squamous histology
Pemetrexed is a multi-targeted anti-folate employed: with platinum derivates for first-line treatment, as single agent for subsequent lines of treatment, and as maintenance therapy. In the landmark JMDB trial, Scagliotti et al. demonstrated no difference in overall survival between cisplatin/gemcitabine and cisplatin/pemetrexed as first-line treatment of patients with metastatic NSCLC. However, in a preplanned subset analysis the cisplatin-pemetrexed combination was superior in non-squamous histology with a median overall survival of 12.6 months in the cisplatin-pemetrexed arm and 10.9 months in the cisplatin-gemcitabine arm (HR =0.84; 95% CI: 0.71-0.99; P=0.03) (13). By contrast, patients with squamous carcinoma had a worse median overall survival in the cisplatin-pemetrexed arm than in the cisplatin-gemcitabine arm (9.4 vs. 10.8 months; HR =1.23; 95% CI: 1.0-1.5; P=0.05).
In a more recent study the Norwegian Lung Cancer Study Group enrolled 436 patients to compare health-related quality of life (HRQoL) between carboplatin-pemetrexed and carboplatin-gemcitabine as first-line treatments for advanced NSCLC. The two regimens achieved similar results in terms of HRQoL and overall survival (7.3 months for carboplatin-pemetrexed vs. 7.0 months for carboplatin-gemcitabine; P=0.63) (14). Multivariate analyses and interaction tests did not reveal any significant associations between specific histology and survival. Carboplatin-pemetrexed combination was not superior in non-squamous histology, in contrast to the JMDB trial. In another randomized phase III trial carboplatin-pemetrexed achieved a longer median survival without toxicity when compared to carboplatin-docetaxel in advanced non-squamous NSCLC (3.2 vs. 0.7 months; HR =0.45; 95% CI: 0.34-0.61). The primary end-point of survival without toxicity was defined as the interval from randomization to the first treatment-induced grade 3-4 adverse event (15). In a meta-analysis published in 2012, Li and colleagues evaluated a selection of clinical trials in which platinum-based combinations including pemetrexed were compared with platinum-based combinations including other third- generation agents for first-line treatment. A consistent survival advantage with pemetrexed was observed especially in non-squamous NSCLC (which represented the majority of the patients) (16). A meta-analysis of five trials (three first-line trials, one second-line trial, one maintenance trial) confirmed that pemetrexed, when compared with alternative treatments or placebo, is consistently associated with a significant overall survival improvement in non-squamous histology (HR =0.82) but not in squamous histology (HR =1.19) (17).
Combining chemotherapy with targeted agents
The diagnosis and management paradigm of metastatic NSCLC has transitioned into an algorithm of presence or absence of oncogene addiction as a key branch point to selecting appropriate treatment. As described above, with the identification of driver mutations such as EGFR and ALK, EGFR-TKIs and crizotinib are supplanting traditional chemotherapy for upfront treatment of these patients (18). However, initial TKI responders inevitably relapse due to acquired resistance. More recently, an added layer of complexity related to intrapatient tumor heterogeneity has been observed, particularly relevant to the clonal evolution of somatic mutations from the primary tumor to metastatic lesions and the mixed response to treatment in different tumor sites (2). At the same time, chemotherapy combinations have reached a therapeutic plateau for metastatic disease (19). Therefore, an area of focus has therefore been on interrogating the combination of novel targeted agents together with chemotherapy to optimize efficacy, survival and overcome acquired resistance. Early studies done combining EGFR-inhibitors with concurrent chemotherapy in unselected populations did not confer a survival advantage (20).
Given the lack of benefit seen in combining concurrent chemotherapy and EGFR TKIs in an unselected patient population, efforts to best integrate chemotherapy and TKI regimens are ongoing. One such approach is intercalating a TKI with chemotherapy based on the preclinical rationale that EGFR TKIs cause G1 cell-cycle arrest thus inhibiting cell-cycle dependent cytotoxic effects of chemotherapy (21). Because the mechanism of action of EGFR-TKIs has the theoretical potential to interfere with or even negate the effects of chemotherapy, it has been hypothesized that sequential or intermittent schedules to confer pharmacodynamic separation may confer better benefit (18).
Table 1 lists recent phase III trial results combining chemotherapy with a targeted agent or novel small molecule inhibitors for within the past two years. The treatment algorithms include single-target agents, multi-target agents, concurrently, intercalated with chemotherapy and as maintenance.
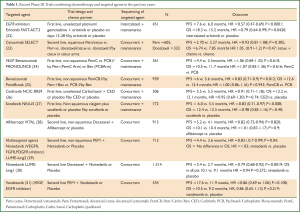
Full Table
The recently published FASTACT-2 study shows that intercalating erlotinib and chemotherapy yields improved progression-free survival and overall survival in East Asian patients enriched for EGFR-activating mutations. However, progression-free survival and overall survival were not significantly different in EGFR wild-types groups (22). Treatment benefit was noted only in patients whose tumors harbored an EGFR activating mutation (median progression-free survival 16.8 vs. 6.9 months, HR =0.25; P<0.0001; median overall survival 31.4 vs. 20.6 months, HR =0.48; P=0.0092).
The anti-VEGF monoclonal antibody Bevacizumab has a demonstrated overall survival benefit in combination with carboplatin and paclitaxel in a phase III trial and this combination can be considered an option in treating nonsquamous NSCLC. However, since the year 2000, over 11 other phase III trials have been negative to date for an overall survival benefit when combining bevacizumab or other anti-angiogenic agents to platinum based chemotherapies. One important issue in employing anti-angiogenesis therapy is absence of a predictive marker for therapeutic benefit. Differences in progression-free survival vs. overall survival benefits may also be confounded by effect of further therapies, given the existence of a variety of moderately active agents now available for second and third line treatments.
In the recent PRONOUCE study the primary objective was to compare progression-free survival without Grade 4 toxicity (G4PFS) between a two drug regimen (Pem/Carbo) vs. three (Pac/Carbo + Bev) in a phase III superiority trial (24). The rationale for this trial design can be questioned. Nevertheless, study outcomes were negative. In the PointBreak trial patients were randomized to carboplatin/paclitaxel/bevacizumab followed by bevacizumab maintenance and compared to carboplatin/pemetrexed/bevacizumab followed by pemetrexed/bevacizumab maintenance (25). There was no overall survival advantage inclusive of all age subgroups. In fact, OS was numerically in favor of paclitaxel.
Nintedanib is a novel multitargeted oral inhibitor of VEGFR, FGFR, and PDGFR, which showed improved progression-free survival when combined with chemotherapy (29,30). Other exploratory avenues showing early signals in combination with chemotherapy include combining immunotherapies such as Ipilumumab or PD-L1 or chaperone proteins such as Hsp90 inhibitor Ganetespib (32-34).
In summary, optimal methods for combing chemotherapy and targeted therapies remain unclear. In addition, these trials emphasize that patient selection factors may dictate outcomes independent of the therapies being evaluated.
Maintenance therapy in advanced NSCLC
Maintenance therapy strategies that improve patient outcomes are an area of active investigation in NSCLC. Both continuation and switch maintenance approaches have been actively studied. Continuation maintenance strategies hope to suppress tumor growth beyond the time of 4 cycles of standard front-line chemotherapy. Alternatively, switch maintenance strategies hope to delay resistance to treatment by incorporating a new chemotherapeutic agent with a different mechanism of action. Ultimately, the goal of maintenance therapy is not just enhance progression-free survival, but to prolong overall survival without decreasing QoL.
The most prominent recently published study of maintenance chemotherapy is PARAMOUNT. In this large, phase III trial patients with non-squamous NSCLC were randomized to pemetrexed or placebo plus best supportive care after induction with 4 cycles of cisplatin/pemetrexed. Both progression-free (HR =0.62, P<0.0001) and overall survival (HR =0.78, P=0.019) were significantly prolonged with continuation maintenance pemetrexed (35,36). Discontinuation of maintenance pemetrexed due to toxicity was low (5%). A comparable number of patients in both treatment arms received post-discontinuation therapy (64% of patients treated with placebo and 58% of patients treated with pemetrexed maintenance). However, maintenance therapy is expensive. A recent Chinese cost-effectiveness analysis estimated cost per quality adjusted life year of maintenance pemetrexed in the Chinese health care system to be between $125,000 and $180,000 (37). Furthermore, it remains unclear in non-squamous patients whether close follow up with timely second line therapy or re-initiation of pemetrexed upon progression would have comparable efficacy to pemetrexed maintenance, particularly in patients who initially benefit from a first-line platinum/pemetrexed doublet, and then are observed without maintenance. Lastly, there is considerable debate as to whether 4 cycles of induction chemotherapy is an adequate point for consideration of maintenance, or whether the 2 months increase in median PFS could be achieved with further induction therapy.
In another pemetrexed maintenance trial (JMEN) that used a switch maintenance strategy, overall survival was improved and patients’ QoL was similar compared to placebo except for a slight decrease in appetite and delayed worsening of hemoptysis and pain (38). In particular, the results of this trial are confounded by a very low rate of second line crossover to pemetrexed in the placebo arm, making real world interpretation difficult. Other trials employing maintenance with gemcitabine and docetaxel after frontline chemotherapy did not show any overall survival benefit when compared to initiating treatment after progression of disease (39,40). A criticism of many maintenance trials is the high percentage of patients randomized to the best-supportive care only arm failing to receive second-line therapy upon progression. Subset analyses of some maintenance treatment trials suggest that patients with stable disease may benefit more from a maintenance strategy, rather than those who respond. Though hypothesis generating, the rationale is sound: patients who do not have a response may progress quicker and would typically receive early second line agents. Thus, regardless of terminology, a switch to docetaxel or gemcitabine could be considered second line therapy instead of maintenance therapy, particularly in squamous histology patients with good functional status who do not have a response to frontline therapy.
New chemotherapeutics
Albumin-bound paclitaxel
Taxanes have been a backbone of NSCLC therapy for well over a decade. 130-nm albumin bound paclitaxel (nab-paclitaxel) differs from standard bound paclitaxel (sb-paclitaxel) by being preferentially taken up into cancer cells via caveolae mediated transcytosis. The proposed mechanism involves enhanced drug delivery to tumor by albumin binding to SPARC (secreted protein, acidic and rich in cysteine), which is preferentially expressed on tumor cells compared to normal tissue (41). It also lacks the cremophor vehicle present in standard bound paclitaxel that can trigger allergic reactions. Nab-paclitaxel was studied in combination with carboplatin and compared to sb-paclitaxel plus carboplatin as first-line therapy of metastatic NSCLC in a large, randomized phase III trial. This trial met its primary endpoint of increased response rate for the carboplatin and nab-paclitaxel combination (33% vs. 25%, P=0.005) (42). The largest gains in response rates were noted in squamous cell histology patients (41% vs. 24%) and no increase in ORR was seen in non-squamous histology. There also was less grade ≥3 neuropathy compared to the sb-paclitaxel combination. However, no significant improvement in overall or progression free survival was noted. In a subset analysis, patients from North America and age ≥70 had significantly improved overall survival with nab-paclitaxel, however this subset analysis should be considered hypothesis generating only. Nab-paclitaxel is clearly a suitable substitute for sb-paclitaxel when allergy to the cremophor vehicle is present or in patients with baseline neuropathy. In addition, nab-paclitaxel could be considered preferential in those with squamous histology when a response is needed, where a subset analysis showed a higher difference in response rates. This rationale is also supported by the realization that new treatment options for NSCLC patients with squamous histology lag far behind those for lung adenocarcinoma.
Cabazitaxel
Cabazitaxel is another taxane currently being studied in a phase II trial in advanced NSCLC (NCT01438307). Recent data in metastatic prostate cancer that showed a significant overall survival benefit underlies the merit of its evaluation in NSCLC (43). Trial results with cabazitaxel in metastatic NSCLC are not yet mature.
Vintafolide (EC145): a folate-vinca alkaloid conjugate
Vinca alkaloids have documented activity in NSCLC, but have largely been supplanted by taxanes and pemetrexed for first or second line systemic treatment of NSCLC. Vintafolide is a conjugate folate molecule linked to vinblastine. Over 75% of NSCLC is folate receptor positive (by immunohistochemistry), offering the potential of folate receptor-targeted therapy. In a recent phase II trial, companion imaging of the folate receptor via 99Tc-EC20 CT scans was used to select patients with folate receptor expressing tumors for treatment with vintafolide. Thus, EC20 uptake is under development as a potential predictive biomarker to vintafolide. In a phase II trial of heavily pretreated relapsed/refractory NSCLC patients with positive EC20 scans, clinical benefit (stable disease + overall response rate) was seen in 26% of patients (44). Currently vintafolide is being studied in combination with docetaxel in a randomized phase II trial of relapsed/refractory NSCLC patients (NCT01577654).
Eribulin mesylate
Eribulin mesylate is a synthetic analogue of halichondron B isolated from a rare marine sponge. It inhibits microtubule dynamics using a distinct mechanism from taxanes or vinca alkaloids. It was recently approved for breast cancer based on a trial showing improved overall survival in heavily pre-treated metastatic breast cancer patients who previously received an anthracycline and a taxane (45). In a phase II trial in NSCLC patients who had previously received a taxane, response rates were low (5%), but 50% of patients achieved stable disease (46). Eribulin is currently being studied in combination with erlotinib (NCT01104155), pemetrexed (NCT01126736) or physicians choice of control drug (NCT01454934) in 3 separate clinical trials.
Ixabepilone
Ixabepilone is an epithilone (a novel anti-microtubule class of agent) that similar to taxanes binds and stabilizes microtubules, eventually resulting in G2/M cell-cycle arrest. Some preclinical studies show it is active in taxane-resistant models and ixabepilone is approved for treatment of metastatic breast cancer. In a randomized phase II trial in NSCLC, it did not improve overall survival or achieve any other clinically meaningful endpoint (47). The investigators stratified patients based on beta-3 tubulin immunohistochemistry and showed it to be a negative prognostic indicator, but not a predictive marker of benefit to ixabepilone. As there is no clear signal of superiority compared to paclitaxel, the future development of ixabepilone in advanced NSCLC treatment is unclear.
Pralatrexate
Pralatrexate, a folate analogue targeting dihydrofolate reductase, was recently studied in a randomized phase II trial compared to erlotinib in metastatic NSCLC patients who progressed on first-line therapy. A trend towards increased overall survival was observed and an increase in progression free survival was noted (48). In this study 18 of 100 patients treated with pralatrexate had prior pemetrexed. There was a high rate of mucositis with pralatrexate despite B12 and folic acid supplementation. As pemetrexed is increasingly being incorporated into upfront treatment regimens of non-squamous NSCLC and the toxicity of pralatrexate appears higher, the role of additional anti-folate therapies is unclear.
Summary of new chemotherapeutic agents
Multiple new chemotherapeutic agents are currently in clinical development or have been recently evaluated in NSCLC (Table 2). Several of these drugs are from similar drug classes to those already shown to be active in NSCLC (cabazitaxel, pralatrexate) while others have been reformulated to preferentially target tumor cells (albumin-bound paclitaxel, vintafolide). Ixabepilone and eribulin affect microtubule dynamics through distinct mechanisms of action compared to taxanes. None of the clinical trials to date with these drugs suggest dramatic benefits in advanced NSCLC patients, but some of these new agents may have a role in specific treatment settings, as per nab-paclitaxel discussed above.
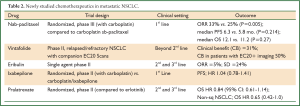
Full Table
Discussion
Chemotherapy remains the indispensible choice for the vast majority of patients with advanced NSCLC, given the relative rarity of currently defined and treatable oncogene-driven patient subsets. Several new chemotherapeutic agents for NSCLC are in clinical development, though their actual role in the current treatment paradigm is yet to be determined. As we seek to rank, order and rationally combine existing chemotherapies to achieve optimal patient outcomes, some promising results have emerged. Switch or continuation maintenance strategies are of benefit, but defining exactly who to treat remains problematic, as the trial designs may not have always reflected real-world considerations. Several aspects of maintenance therapy need further examination including the optimal number of induction chemotherapy cycles, the role of treatment-free intervals, QoL, economic considerations, and whether progression-free survival is a worthy therapeutic goal in this disease setting (49). Platinum based cytotoxic chemotherapy has been the backbone of treatment for metastatic NSCLC for decades and non-platinum combinations have not shown superiority. Attempts to employ biomarkers of DNA repair or other biomarkers for chemotherapy have been hindered by methodological issues to date. Optimal strategies for integrating chemotherapy and targeted therapeutics are an area of active investigation with promising results.
Despite the remarkable advances in the targeted treatment of NSCLC in the past several years, chemotherapy remains of paramount importance in the treatment of advanced NSCLC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s Lung Cancer Mutation Consortium (LCMC). J Clin Oncol 2011;29:abstr CRA7506.
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [PubMed]
- Jiang J, Liang X, Zhou X, et al. Non-platinum doublets were as effective as platinum-based doublets for chemotherapy-naïve advanced non-small-cell lung cancer in the era of third-generation agents. J Cancer Res Clin Oncol 2013;139:25-38. [PubMed]
- Zukin M, Barrios CH, Rodrigues Pereira J, et al. Randomized Phase III Trial of Single-Agent Pemetrexed Versus Carboplatin and Pemetrexed in Patients With Advanced Non-Small-Cell Lung Cancer and Eastern Cooperative Oncology Group Performance Status of 2. J Clin Oncol 2013;31:2849-53. [PubMed]
- Altaha R, Liang X, Yu JJ, et al. Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med 2004;14:959-70. [PubMed]
- Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol 2006;24:4731-7. [PubMed]
- Bepler G, Williams C, Schell MJ, et al. Randomized International Phase III Trial of ERCC1 and RRM1 Expression-Based Chemotherapy Versus Gemcitabine/Carboplatin in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2013;31:2404-12. [PubMed]
- Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 2013;368:1101-10. [PubMed]
- Sigmond J, Backus HH, Wouters D, et al. Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol 2003;66:431-8. [PubMed]
- Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 2005;68:110-8. [PubMed]
- Maus MK, Mack PC, Astrow SH, et al. Histology-related associations of ERCC1, RRM1, and TS biomarkers in patients with non-small-cell lung cancer: implications for therapy. J Thorac Oncol 2013;8:582-6. [PubMed]
- Shao H, Tang H, Salavaggione OE, et al. Improved response to nab-paclitaxel compared with cremophor-solubilized paclitaxel is independent of secreted protein acidic and rich in cysteine expression in non-small cell lung cancer. J Thorac Oncol 2011;6:998-1005. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Grønberg BH, Bremnes RM, Fløtten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217-24. [PubMed]
- Rodrigues-Pereira J, Kim JH, Magallanes M, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:1907-14. [PubMed]
- Li M, Zhang Q, Fu P, et al. Pemetrexed plus platinum as the first-line treatment option for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. PLoS One 2012;7:e37229. [PubMed]
- Al-Saleh K, Quinton C, Ellis PM. Role of pemetrexed in advanced non-small-cell lung cancer: meta-analysis of randomized controlled trials, with histology subgroup analysis. Curr Oncol 2012;19:e9-e15. [PubMed]
- Pennell NA. Integration of EGFR inhibitors and conventional chemotherapy in the treatment of non-small-cell lung cancer. Clin Lung Cancer 2011;12:350-9. [PubMed]
- Belani CP, Goss G, Blumenschein G Jr. Recent clinical developments and rationale for combining targeted agents in non-small cell lung cancer (NSCLC). Cancer Treat Rev 2012;38:173-84. [PubMed]
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892-9. [PubMed]
- Gandara DR, Gumerlock PH. Epidermal growth factor receptor tyrosine kinase inhibitors plus chemotherapy: case closed or is the jury still out? J Clin Oncol 2005;23:5856-8. [PubMed]
- Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [PubMed]
- Kim ES, Neubauer MA, Cohn AL, et al. SELECT: Randomized phase III study of docetaxel (D) or pemetrexed (P) with or without cetuximab (C) in recurrent or progressive non-small cell lung cancer (NSCLC) after platinum-based therapy. J Clin Oncol 2012;30:abstr 7502.
- Zinner R, Ross HJ, Weaver R, et al. Randomized, open-label, phase III study of pemetrexed plus carboplatin (PemC) followed by maintenance pemetrexed versus paclitaxel/carboplatin/bevacizumab (PCB) followed by maintenance bevacizumab in patients with advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr LBA8003.
- Socinski MA, Patel JD, Garon EB, et al. A phase III study of pemetrexed (Pem) plus carboplatin (Cb) plus bevacizumab (Bev) followed by maintenance pem plus bev versus paclitaxel (Pac) plus cb plus bev followed by maintenance bev in stage IIIb or IV nonsquamous non-small cell lung cancer (NS-NSCLC): overall and age group results. J Clin Oncol 2013;31:abstr 8004.
- Laurie SA, Solomon BJ, Seymour L, et al. A randomized double-blind trial of carboplatin plus paclitaxel (CP) with daily oral cediranib (CED), an inhibitor of vascular endothelial growth factor receptors, or placebo (PLA) in patients (pts) with previously untreated advanced non-small cell lung cancer (NSCLC): NCIC Clinical Trials Group study BR29. J Clin Oncol 2012;30:abstr 7511.
- Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 2012;30:3084-92. [PubMed]
- Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7. [PubMed]
- Hanna NH, Kaiser R, Sullivan RN, et al. Lume-lung 2: a multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy. J Clin Oncol 2013;31:abstr 8034.
- Reck M, Kaiser R, Mellemgaard A, et al. Nintedanib (BIBF 1120) plus docetaxel in NSCLC patients progressing after first-line chemotherapy: LUME Lung 1, a randomized, double-blind phase III tria. J Clin Oncol 2013;31:abstr LBA8011.
- de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011;29:1067-74. [PubMed]
- Tomasini P, Khobta N, Greillier L, et al. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol 2012;4:43-50. [PubMed]
- Fennell DA, Goss DG, Socinski MA, et al. GALAXY-2 trial: a randomized phase III study of ganetespib in combination with docetaxel versus docetaxel alone in patients with advanced non-small cell lung adenocarcinoma. J Clin Oncol 2013;31:abstr TPS8126.
- Spigel DR, S NG, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:abstr 8008.
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [PubMed]
- Paz-Ares L, F DM, Dediu M, et al. PARAMOUNT: Final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr LBA7507.
- Zeng X, Peng L, Li J, et al. Cost-effectiveness of continuation maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for advanced nonsquamous non-small-cell lung cancer: estimates from the perspective of the Chinese health care system. Clin Ther 2013;35:54-65. [PubMed]
- Belani CP, Brodowicz T, Ciuleanu TE, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol 2012;13:292-9. [PubMed]
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8. [PubMed]
- Belani CP, Waterhouse DM, Ghazal H, et al. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:15:abstr 7506
- Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother 2006;7:1041-53. [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [PubMed]
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147-54. [PubMed]
- Edelman MJ, Harb WA, Pal SE, et al. Multicenter trial of EC145 in advanced, folate-receptor positive adenocarcinoma of the lung. J Thorac Oncol 2012;7:1618-21. [PubMed]
- Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011;377:914-23. [PubMed]
- Gitlitz BJ, Tsao-Wei DD, Groshen S, et al. A phase II study of halichondrin B analog eribulin mesylate (E7389) in patients with advanced non-small cell lung cancer previously treated with a taxane: a California cancer consortium trial. J Thorac Oncol 2012;7:574-8. [PubMed]
- Edelman MJ, Schneider CP, Tsai CM, et al. Randomized phase II study of ixabepilone or paclitaxel plus carboplatin in patients with non-small-cell lung cancer prospectively stratified by beta-3 tubulin status. J Clin Oncol 2013;31:1990-6. [PubMed]
- Kelly K, Azzoli CG, Zatloukal P, et al. Randomized phase 2b study of pralatrexate versus erlotinib in patients with stage IIIB/IV non-small-cell lung cancer (NSCLC) after failure of prior platinum-based therapy. J Thorac Oncol 2012;7:1041-8. [PubMed]
- Gerber DE, Schiller JH. Maintenance chemotherapy for advanced non-small-cell lung cancer: new life for an old idea. J Clin Oncol 2013;31:1009-20. [PubMed]


