Safe administration of intrapleural alteplase during pregnancy
Introduction
Parapneumonic pleural effusions are a common complication of pneumonia, occurring in up to 40% of patients who are hospitalized for pneumonia in the United States each year (1,2). Parapneumonic pleural effusions are most commonly a complication of bacterial pneumonias, especially Streptococcus pneumonia, whereas approximately 20% of patients with viral or Mycoplasma pneumonia develop pleural effusions (1,2). Patients with pneumonia who develop a parapneumonic pleural effusion have a higher rate of mortality than those patients with pneumonia who do not (2). Of the patients diagnosed with pleural infections each year, approximately 15% die and 15–40% require surgical intervention within weeks of intercostal chest tube placement (3-5). Furthermore, the economic burden is high with more than $300 million dollars spent each year to manage patients with parapneumonic pleural effusions and empyemas (3,5,6).
Instillation of intrapleural (IP) fibrinolytics has been used in patients with complicated parapneumonic pleural effusions with the intention to improve pleural fluid drainage and avoid surgical intervention such as surgical debridement of the pleural space (7,8). Data supporting this practice in adult patients has mixed results and is currently not approved by the Food and Drug Administration (FDA) for this indication. The MIST1 trial and a meta-analysis failed to demonstrate a reduction in surgical interventions with IP streptokinase (5,9). Subsequently, the MIST2 trial showed that patients who have only received IP tissue plasminogen activator (tPA) did not demonstrate a reduction in surgical interventions compared to placebo (10). However, the addition of IP recombinant human DNase to IP tPA improved chest tube drainage, decreased the need for surgical intervention and shortened hospital length of stay (10). Recently, Nie et al. showed in a meta-analysis that IP fibrinolytics may reduce the need for surgery and thus decrease hospital length of stay without additional harm to patients with parapneumonic pleural effusions (11).
Given the mixed results regarding the effectiveness of IP administration of fibrinolytics for parapneumonic pleural effusions, the clinical benefits should clearly outweigh the risks. Although, the effects of IP administration of fibrinolytics are localized, there are reports of systemic effects including leukocytosis, fatigue, fever, and bleeding (2). Pregnant women are at increased risk of thromboembolic diseases, especially venous thromboembolism, since pregnancy is a state of relative hypercoagulability, peaking in the third trimester and peripartum period (12). Case reports have been published describing the use of systemic fibrinolytic therapy in pregnant patients for indications such as ischemic stroke, thrombosis of prosthetic cardiac valve, venous thrombotic events, and myocardial infarction, with minimal maternal and fetal complications and no reports of teratogenicity (13-16). However, clinical trials have not evaluated IP fibrinolytics in management of parapneumonic pleural effusions in pregnant patients, and there are currently no published cases describing the use of IP fibrinolytic therapy for the management of complicated parapneumonic pleural effusions in these patients.
Case presentation
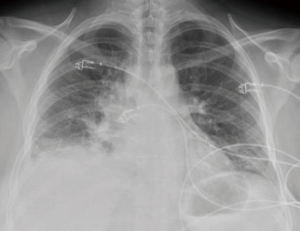
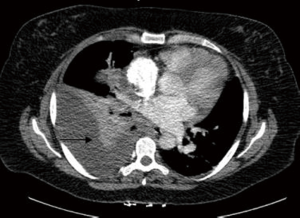
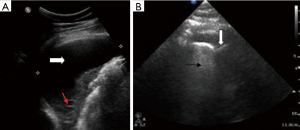
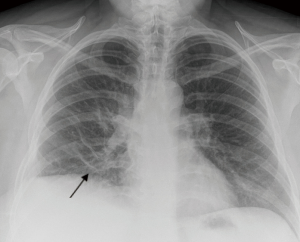
We describe the case of a 35-year-old female, G2P1 at 32 weeks of gestation, who presented with cough, dyspnea, and right sided pleuritic chest pain. Her symptoms started five days prior to hospital admission and did not respond to empiric oral antibiotic therapy. On examination, she was afebrile and hemodynamically stable with no respiratory distress. Laboratory evaluation was notable for leukocytosis (22.10 K/µL), but otherwise normal. Chest radiograph revealed right sided pulmonary infiltrates (Figure 1). Given her increased oxygen requirements from baseline, she underwent a computed tomography scan of the chest to rule out a pulmonary embolism, which subsequently showed a right lower lobe consolidation and an ipsilateral loculated pleural effusion (Figure 2). A chest ultrasound measured a 14 cm × 9.5 cm × 6.1 cm pleural effusion with an approximate volume of 811 mL (Figure 3A). Her rapid influenza testing was positive for influenza A and sputum culture grew group A Streptococcus, thus she was treated for influenza pneumonia with superimposed bacterial pneumonia with oseltamivir and penicillin G respectively. Given concern for an infected pleural space, an ultrasound guided thoracentesis was performed. Pleural fluid studies showed the following: pH 7.51, glucose 12 mg/dL, LDH 3,619 U/L, and total protein 5 g/dL, indicating a complicated parapneumonic pleural effusion. Pleural fluid cultures were negative. Subsequently, she underwent percutaneous placement of a 14 French intercostal chest tube with initial drainage of 300 mL of non-purulent, straw colored fluid. Repeat imaging showed persistent pleural effusion with no further drainage from the chest tube. Thus, we considered the administration of IP tPA to improve chest tube output after reviewing the potential risks of the procedure with the patient including placental bleeding, IP hematoma, hemorrhage, and chest pain. A dose of 2.5 mg of tPA was initially administered through the chest tube and was left to dwell in the pleural space for two hours while a nurse assisted the patient turning sides every 30 minutes. Subsequently, the chest tube was placed to wall suction at −20 cmH2O, with an additional 250 mL of pleural fluid drained. The patient tolerated the procedure well with no immediate complications. Repeat chest radiographs and ultrasound showed significant improvement in the size of the residual pleural effusion (Figures 3B,4). No further IP tPA was required. The chest tube was eventually removed without complication four days post-IP tPA administration. Cultures from the pleural fluid remained negative. The patient was successfully discharged to complete a 2-week course of amoxicillin/clavulanic acid. Upon outpatient follow-up, patient had an uneventful delivery at 40 weeks of gestation with no adverse sequelae noted in the newborn.
Due to concern for possible adverse outcomes such as premature labor, placental bleeding, placental abruption and fetal demise, pregnancy continues to be a relative contraindication for thrombolysis (17). In fact, all randomized control trials looking at tPA evaluating tPA for thrombolysis have excluded pregnant women. All data we have in this high-risk population comes from cases reports and case series. Interestingly, to date, there has been no observed cases of teratogenicity or increased fetal risk with use of systemic tPA in pregnancy. In addition, all reported cases of fetal and maternal deaths were attributed to severity of underlying medical conditions rather than a complication from use of thrombolytics and bleeding rates were comparable to overall risk in the general population (13-16).
Many molecular properties of medications including protein binding, pH, and molecular weight, must be considered when evaluating their ability to cross the placenta. Drugs with a molecular weight greater than 1,000 Da are unlikely to cross into the placenta (18). Therefore, tPA has low teratogenicity risk since it is unlikely to cross the placenta with a molecular weight of 7,200 Da (12). In addition, the molecule has high affinity for fibrin strands and a short half-life of 4–5 minutes via hepatic metabolism, with only 10% of its concentration remaining after 20 minutes, which explains its low complication rates. Based on current available literature, the FDA designated tPA as pregnancy category C, indicating that animal studies have shown harm to the fetus and there are no well controlled human studies evaluating tPA in pregnant women (17). Therefore, due to absence of randomized controlled trials, the decision to use tPA during pregnancy should be made on a case-by-case basis.
Herein, we report the first use of IP tPA for management of a complicated parapneumonic pleural effusion in a pregnant patient. Our patient was successfully treated with a lower dose of IP tPA compared to doses studied in previous trials (5,10,11) with no complications experienced by either her or her fetus, although it is important to note that our patient was late in her third trimester of pregnancy. We believe that administration of IP tPA spared our patient the need for more invasive surgical procedures such as video-assisted thoracoscopic surgical decortication which might increase morbidity. Despite our successful experience, we still recommend careful consideration of the risks prior to administration of IP tPA for management of a complicated parapneumonic pleural effusion in the setting of pregnancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007;45:1480-6. [Crossref] [PubMed]
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Davies CW, Kearney SE, Gleeson FV, et al. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med 1999;160:1682-7. [Crossref] [PubMed]
- Maslove DM, Chen BT, Wang H, et al. The diagnosis and management of pleural effusions in the ICU. J Intensive Care Med 2013;28:24-36. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled Trial of Intrapleural Streptokinase for Pleural Infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Netten A, Dennett J, Knight J. Unit costs of health and social care 1999. Canterbury, UK: University of Kent, 1999.
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52:416-21. [Crossref] [PubMed]
- Bouros D, Schiza S, Patsourakis G, et al. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions: a prospective, double-blind study. Am J Respir Crit Care Med 1997;155:291-5. [Crossref] [PubMed]
- Tokuda Y, Matsushima D, Stein GH, et al. Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: a meta-analysis. Chest 2006;129:783-90. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural Use of Tissue Plasminogen Activator and DNase in Pleural Infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Nie W, Liu Y, Ye J, et al. Efficacy of intrapleural instillation of fibrinolytics for treating pleural empyema and parapneumonic effusion: a meta-analysis of randomized control trials. Clin Respir J 2014;8:281-91. [Crossref] [PubMed]
- Gartman EJ. The use of thrombolytic therapy in pregnancy. Obstet Med 2013;6:105-11. [Crossref] [PubMed]
- Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271-6. [Crossref] [PubMed]
- Li Y, Margraf J, Kluck B, et al. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review. Neurologist 2012;18:44-8. [Crossref] [PubMed]
- Tversky S, Libman RB, Reppucci ML, et al. Thrombolysis for Ischemic Stroke during Pregnancy: A Case Report and Review of the Literature. J Stroke Cerebrovasc Dis 2016;25:e167-70. [Crossref] [PubMed]
- Steinberg A, Moreira TP. Neuroendocrinal, Neurodevelopmental, and Embryotoxic Effects of Recombinant Tissue Plasminogen Activator Treatment for Pregnant Women with Acute Ischemic Stroke. Front Neurosci 2016;10:51. [Crossref] [PubMed]
- Activase® (alteplase) for injection [prescribing information]. San Francisco, CA: Genentech, Inc; 2015. Available online: https://www.gene.com/download/pdf/activase_prescribing.pdf. Accessed June 19, 2017.
- Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 2004;43:487-514. [Crossref] [PubMed]


