Features of rheumatic mitral valves and a grading system to identify suitable repair cases in China
Introduction
Rheumatic heart disease affects approximately 2 million adult Chinese patients and has a prevalence of 2% (1). According to statistics from a database from Anzhen Hospital Cardiac Valve Centre, among 7,952 patients who underwent mitral valve operation, 3,221 (40.5%) suffered from rheumatic disease in the last 20 years. Among most Chinese surgeons, the procedure of choice to treat rheumatic heart disease is mitral valve replacement (MVR) (2). The feasibility and durability of rheumatic mitral valve repair (rMVP), another procedure to treat rheumatic heart disease, were confirmed using the mid-to-long-term follow-up results of foreign studies (3-5). Nevertheless, there are many discrepancies between the pathological characteristics described in those studies and those described for Chinese patients. For example, the preoperative pathology was regurgitation in most cases, and the ratio of stenosis and mixed lesion was low (3,6,7). The proportion of women was low (3), and the majority of patients were young and middle-aged (8,9). Meanwhile, the pathological characteristics of rheumatic mitral valve disease in Chinese patients have not been thoroughly evaluated.
Rheumatic heart disease is still the most common cause of valve disease in China. Therefore, we aimed to describe and analyse the pathological characteristics of rheumatic mitral valve disease in Chinese patients and to formulate a pathological grading system to clearly identify cases that are suitable to undergo rMVP under the condition of current surgical techniques of mitral repair.
Methods
Patient data and endpoints
Data from patients who underwent rMVP between January 2012 and June 2016 were collected. The main endpoints were re-operation or death in the follow-up period. The secondary endpoint was more than moderate mitral stenosis or regurgitation in the follow-up period. Data from consecutive patients who underwent mitral valve repair (MVP) or MVR for rheumatic disease between September 2015 and June 2016 were collected to evaluate the pathological characteristics of rheumatic mitral valve disease. Based on these data, a pathological grading system of rheumatic mitral valve lesion (PGSRMVL) was developed (Table 1). The system contains the following three main parts: the leaflet, commissure, and sub-valvular apparatus. Each grade was defined in detail. Based on the clinical experience of our team, we chose to classify it this way. All evaluation results were based on visual observation.

Full table
All study participants provided informed consent. The study design was approved by the ethics review board of the Capital Medical University affiliated Beijing Anzhen Hospital (No. 857-3).
Statistical analysis
Continuous variables are expressed as means ± standard deviations. Discrete variables are described as percentages. Univariate analysis of continuous variables was carried out with Student’s t-test. Univariate analysis of categorical data was carried out with the χ2 or Fisher’s exact tests. Analysis of freedom from reoperation and valve failure was performed with the Kaplan-Meier survival curve. The PGSRMVL was tested using receiver operating characteristics (ROC) curve analysis. Cox regression analyses were used to determine the risk factors for the endpoints. SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
From January 2012 to June 2016, 179 patients underwent mitral repair for rheumatic disease in Anzhen Hospital Cardiac Valve Centre. The mean age was 52.08±11.89 years. The percentage of female patients was 74.30%. Preoperatively, persistent atrial fibrillation accounted for 55.87% of cases. Among all 179 rMVP cases, there were 12 cases of repair failure during the operation and 167 cases of successful repair with an intraoperative repair rate of 84.77% (Table 2). Among all surgical techniques, commissurotomy and leaflet thinning accounted for the highest percentage at 90.50% and 70.39%, respectively. The percentages of other techniques were not more than 15% (Table 3).
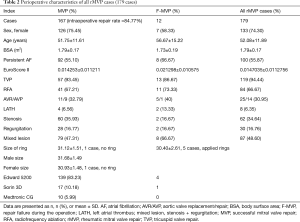
Full table
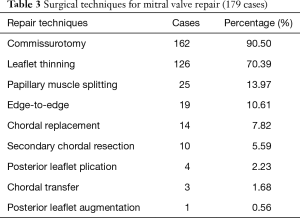
Full table
Data regarding follow-up were obtained until December 2016 and collected during visits to the outpatient clinic or by telephone interviews. The data were 100% complete. The mean follow-up period was 24±14 [6–59] months. In the follow-up period, the main endpoints occurred in 5 patients, including 1 patient who died from intracranial haemorrhage at 1 month after the operation and 4 patients who underwent re-operation (infective endocarditis occurred in 2 patients, and regurgitation occurred in the other 2 patients). The secondary endpoint occurred in 14 patients. Regurgitation occurred in 12 patients among whom 2 required re-operation. Moderate stenosis occurred in 2 patients.
For all 179 cases, the early postoperative echocardiographic indexes (mitral valve orifice area, mitral valve orifice area/body surface area, E-wave, etc.) of the MVP group improved notably compared with the preoperative values. The echocardiographic indexes at 6 months or more than 9 months after the operation were not remarkably different (Table 4). The percentage of freedom from re-operation and valve failure with 5 years in the follow-up period was 96.4%±1.8% and 88.2%±2.9%, respectively. The majority of endpoints occurred within 1 year after the operation (Figure 1).
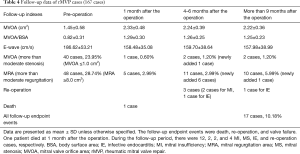
Full table

Univariate and multivariate Cox regression analyses were used to determine the risk factors of 167 patients who underwent MVP for re-operation and valve failure between January 2012 and June 2016. The preoperative left atrial anterior and posterior diameter (LAAPD) >60 mm [hazard ratio (HR) =3.884, P=0.029] was a significant independent predictor (Table 5).
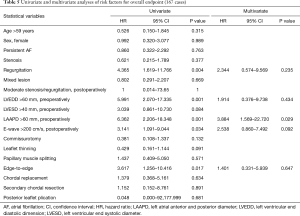
Full table
Between September 2015 and June 2016, 126 consecutive rheumatic mitral valve operations were performed. The number of patients with successful repair and replacement operations was 61 and 65, respectively. The percentage of rMVP cases in our centre was 48.41%. The PGSRMVL was used to analyse the pathological characteristics of rheumatic mitral valve for these 126 cases in our centre. The average score of the 126 cases was 17.28±9.8. The most frequent pathological characteristics were anterior commissural fusion (98.41%), anterior leaflet thickening (95.25%), posterior leaflet thickening (93.65%), posterior commissural fusion (93.65%), and sub-valvular chordae tendineae thickening (76%). The frequencies of severe pathological lesions were as follows: chordae tendineae fusion (45.95%), anterior commissural fusion (41.13%)/calcification (35.42%), chordae tendineae calcification (39.39%), posterior commissural fusion (32.20%)/calcification (36.36%), posterior leaflet thickening (21.19%), and anterior leaflet thickening (10.83%) (Figure 2A).
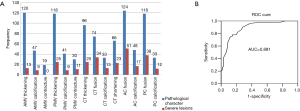
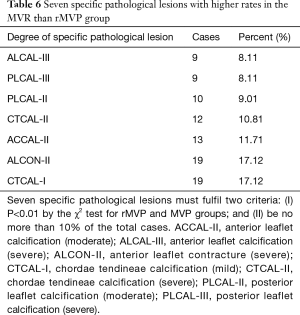
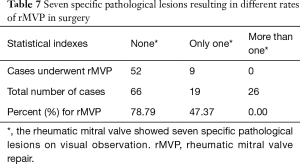
There were no preoperative plans to repair 15 cases and MVR was directly performed, between September 2015 and June 2016. Therefore, 111 cases were analysed. The ROC curve analysis of PGSRMVL for judgment on whether rMVP would be successful showed an ROC area under the curve of 0.891 (cut-off point, 17.5; sensitivity, 78%; specificity, 85.2%) (Figure 2B). The number of patients with a PGSRMVL score <17.5 was 75 (59.52%). All pathological lesions were analysed separately in comparing the MVR group and MVP group. Seven lesions had obviously higher ratios in the MVR group than MVP group. Their P values were less than 0.01 (Table 6). The different number of patient’s lesions can lead to different successful repair ratios. If there was no specific pathological lesion present, the repair rate was 78%. If only one lesion was present, the repair rate was nearly 47%. However, if more than one lesion was present, no repair was accomplished (Table 7).
Full table
Full table
Discussion
Rheumatic heart disease is the most common cause of valve disease in the developing nations (10,11). Chronic inflammation during the rheumatic process may cause narrowing of the valves resulting in decreased blood flow through the heart or leakage of the valves causing blood to flow in the wrong direction. This may eventually lead to arrhythmias such as atrial fibrillation, or heart failure, where the heart is unable to pump enough blood to meet the body’s needs (12). Considering its many advantages such as subvalvular apparatus preservation, protection of left ventricular systolic function (13,14), and avoiding risks of thromboembolism and bleeding resulting from warfarin (15,16), MVP has been acknowledged to be superior to replacement (15,17-19). Several cardiac centres in Asian countries have performed rMVP for decades (20), and have gained a considerable amount of experience (3,4). Because of the complexity and durability of rMVP, the majority Chinese surgeons may choose MVR. However, in recent decades, the exploration of surgical techniques for rMVP has increased in our centre each year.
According to the PGSRMVL used in this study, the most common pathological characteristics of rheumatic mitral valve disease were leaflet thickening, commissural fusion, and sub-valvular chordae tendineae thickening. More than 90% of patients with rheumatic disease were found to have leaflet thickening (anterior 95.25%, posterior 93.65%), commissural fusion (anterior 98.41%, posterior 93.65%), and 76% were found to have sub-valvular chordae tendineae thickening. However, the percentage of patients with severe pathological lesion of the commissure and sub-valvular apparatus was as high as 30–50%, compared with 0–20% for patients with severe pathological lesion of the leaflet. Therefore, the main pathological characteristics of rheumatic mitral valve disease in Chinese patients are lesions of the commissure and sub-valvular apparatus. These results also could explain why the preoperative echocardiographic pathological types in our centre differ from those in other foreign centres, as the percentage of stenosis and mixed lesion cases were notably higher in our centre than in other centres (3,21). Hence, when we formulated the PGSRMVL, we gave those pathological lesions related to sub-valvular apparatus and calcification a higher weight value, based on special pathological characteristics. Because of the particular pathological characteristics shown among our cases, the surgical techniques used in our centre were also different from those of other centres (4,6,13,22-24). The majority of the patients were treated via commissurotomy (90.5%) and thinning (70.39%). Among all 179 rMVP patients, these two techniques were applied to 122 (68.16%) patients, of which 10 underwent MVR and 112 (91.80%) underwent MVP. By the ROC curve analysis for judgment on whether successful rMVP could be achieved, PGSRMVL was proved to clearly predict the complexity and feasibility of rMVP. However, some points need be explained. First, the pathological score system is complicated and difficult to apply in surgery. Although the analysis between pathological score system and rMVP was significant, scoring every patient is not practical during surgery. Second, the key point of this system is to help us understand the distribution of rheumatic mitral valve pathological lesions more clearly and reflect the relationship between specific pathological lesions and difficulty of repair. According to the results (Table 7), we can conclude that severe calcification of the commissure and sub-valvular apparatus can considerably decrease the odds of a successful repair.
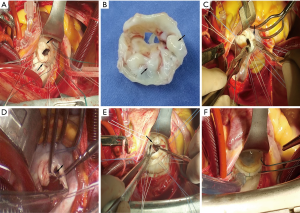
Based on our experience, we have compiled some recommendations for this type of operation. (I) If the leaflet thickening does not affect leaflet distention, the procedure for thinning is meaningless. (II) The scope of leaflet thinning should be suitable, and the procedure should be stopped when necessary. The aim is to recover leaflet distention. (III) Before commissural leaflet thinning, the two sides of the commissure should be hung and the commissure should be in the plane (Figure 3A). (IV) If calcification has involved the full thickness of the leaflet, it is not helpful to recover leaflet distention by leaflet thinning (Figure 3B). (V) Before commissurotomy, thickening and calcification of the commissure should be attended to recover the mobility of the commissure. Subsequently, commissurotomy should be performed based on the nature of the commissure border. (VI) When performing commissurotomy, if it is difficult to detect the sub-valvular apparatus, it should be feasible to cut open a part of commissure close to the annulus first, and then to detect and locate the sub-valvular chordae tendineae (Figure 3C). (VII) A distance of 2–3 mm should be maintained between the cutting end point and annulus (Figure 3D). (VIII) Flexibility of the commissure and sub-valvular apparatus should be verified, during the water test. The middle of the commissure should retract; meanwhile, the leaflet should be distended into the middle from both sides of the commissure. (IX) Papillary muscle splitting should be based on the nature of the papillary muscle (Figure 3E). (X) A sign of good prognosis during the water test is that the mitral valve recovers a ‘smile’ shape (Figure 3F).
With the development of ultrasonic testing technology, especially transesophageal echocardiography (25) and three-dimensional echocardiography, surgeons can plan operations in detail and follow-up on the treatment effect (7). Using multivariate Cox regression analyses for 167 MVP cases, a preoperative LAAPD >60 mm was found to be a significant independent predictor of re-operation and valve failure. A significantly larger left atrial anterior and posterior diameter reflected that the mitral valve lesions had lasted a longer time and the degree of cardiac lesion was more serious; this may also indirectly reflect that the degree of mitral valve lesion was more serious. Therefore, a LAAPD >60 mm can be considered a preoperative predictor of unfavourable prognosis.
Between September 2015 and June 2016, 48.41% patients underwent rMVP in our centre. Considering the large number of patients with rheumatic disease and the low percentage of repair cases, there is still much room for improvement on this subject in China.
This study is limited by its single-centre design and low number of cases. Our findings are susceptible to referral bias and institution-specific practices. Further studies with a longer follow-up period are warranted.
Conclusions
The main pathological characteristics of rheumatic mitral valve disease in Chinese patients are lesions of the commissure and sub-valvular apparatus. Most patients can be appropriately treated via commissurotomy and leaflet thinning. Severe calcification of the commissure and sub-valvular apparatus can considerably decrease the odds of a successful repair. The median follow-up results of rMVP are considered satisfactory for the pathological characteristics in Chinese patients, and the use of this procedure should be considered more frequently among surgeons in China.
Acknowledgements
We thank Professor Yuqing Jiao, Professor Qi Qiu, Professor Kequan Guo, and Physician Assistant Shuping Ding from the Capital Medical University affiliated Beijing Anzhen Hospital for their support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics review board of the Capital Medical University affiliated Beijing Anzhen Hospital (No. 857-3) and written informed consent was obtained from all patients.
References
- Zhimin W, Yubao Z, Lei S, et al. Prevalence of chronic rheumatic heart disease in Chinese adults. Int J Cardiol 2006;107:356-9. [Crossref] [PubMed]
- Wang Z, Zhou C, Gu H, et al. Mitral valve repair versus replacement in patients with rheumatic heart disease. J Heart Valve Dis 2013;22:333-9. [PubMed]
- Dillon J, Yakub MA, Kong PK, et al. Comparative long-term results of mitral valve repair in adults with chronic rheumatic disease and degenerative disease: is repair for "burnt-out" rheumatic disease still inferior to repair for degenerative disease in the current era. J Thorac Cardiovasc Surg 2015;149:771-7; discussion 777-9. [Crossref] [PubMed]
- Yakub MA, Dillon J, Krishna MP, et al. Is rheumatic aetiology a predictor of poor outcome in the current era of mitral valve repair? Contemporary long-term results of mitral valve repair in rheumatic heart disease. Eur J Cardiothorac Surg 2013;44:673-81. [Crossref] [PubMed]
- Bakir I, Onan B, Onan IS, et al. Is rheumatic mitral valve repair still a feasible alternative?: indications, technique, and results. Tex Heart Inst J 2013;40:163-9. [PubMed]
- Antunes MJ. Challenges in rheumatic valvular disease: Surgical strategies for mitral valve preservation. Glob Cardiol Sci Pract 2015;2015:9. [Crossref] [PubMed]
- Kim GS, Lee CH, Kim JB, et al. Echocardiographic evaluation of mitral durability following valve repair in rheumatic mitral valve disease: impact of Maze procedure. J Thorac Cardiovasc Surg 2014;147:247-53. [Crossref] [PubMed]
- Song JK, Kim HS, Song JM, et al. Comparison of clinical and echocardiographic outcomes after valve repair: degenerative versus rheumatic mitral regurgitation. J Korean Med Sci 2003;18:344-8. [Crossref] [PubMed]
- Kalangos A, Christenson JT, Beghetti M, et al. Mitral valve repair for rheumatic valve disease in children: midterm results and impact of the use of a biodegradable mitral ring. Ann Thorac Surg 2008;86:161-8; discussion 168-9. [Crossref] [PubMed]
- Remenyi B, ElGuindy A, Smith SC, et al. Valvular aspects of rheumatic heart disease. Lancet 2016;387:1335-46. [Crossref] [PubMed]
- Marijon E, Mirabel M, Celermajer DS, et al. Rheumatic heart disease. Lancet 2012;379:953-64. [Crossref] [PubMed]
- Harris C, Croce B, Cao C. Rheumatic heart disease. Ann Cardiothorac Surg 2015;4:492. [PubMed]
- Lafci G, Cagli K, Cicek OF, et al. Papillary muscle repositioning as a subvalvular apparatus preservation technique in mitral stenosis patients with normal left ventricular systolic function. Tex Heart Inst J 2014;41:33-9. [Crossref] [PubMed]
- Athanasiou T, Chow A, Rao C, et al. Preservation of the mitral valve apparatus: evidence synthesis and critical reappraisal of surgical techniques. Eur J Cardiothorac Surg 2008;33:391-401. [Crossref] [PubMed]
- Suri RM, Schaff HV, Dearani JA, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82:819-26. [Crossref] [PubMed]
- AlTurki A, Proietti R, Birnie DH, et al. Management of antithrombotic therapy during cardiac implantable device surgery. J Arrhythm 2016;32:163-9. [Crossref] [PubMed]
- McNeely CA, Vassileva CM. Long-term outcomes of mitral valve repair versus replacement for degenerative disease: a systematic review. Curr Cardiol Rev 2015;11:157-62. [Crossref] [PubMed]
- Gaur P, Kaneko T, McGurk S, et al. Mitral valve repair versus replacement in the elderly: short-term and long-term outcomes. J Thorac Cardiovasc Surg 2014;148:1400-6. [Crossref] [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6:S39-51. [PubMed]
- Choudhary SK, Talwar S, Dubey B, et al. Mitral valve repair in a predominantly rheumatic population. Long-term results. Tex Heart Inst J 2001;28:8-15. [PubMed]
- El Oumeiri B, Boodhwani M, Glineur D, et al. Extending the scope of mitral valve repair in rheumatic disease. Ann Thorac Surg 2009;87:1735-40. [Crossref] [PubMed]
- Dillon J, Yakub MA, Nordin MN, et al. Leaflet extension in rheumatic mitral valve reconstruction. Eur J Cardiothorac Surg 2013;44:682-9. [Crossref] [PubMed]
- Chan PG, Hayanga AJ, Badhwar V. Repair of rheumatic mitral stenosis with bicommissural release, anterior leaflet augmentation and oversized annuloplasty. Multimed Man Cardiothorac Surg 2014;2014:mmt020. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Capoulade R, et al. A Systematic Review of Mitral Valve Repair With Autologous Pericardial Leaflet Augmentation for Rheumatic Mitral Regurgitation. Ann Thorac Surg 2016;102:1400-5. [Crossref] [PubMed]
- Liu FZ, Xue YM, Liao HT, et al. Five-year epidemiological survey of valvular heart disease: changes in morbidity, etiological spectrum and management in a cardiovascular center of Southern China. J Thorac Dis 2014;6:1724-30. [PubMed]


