Minimally invasive esophagectomy is a safe surgical treatment for locally advanced pathologic T3 esophageal squamous cell carcinoma
Introduction
Esophageal carcinoma is a relatively common malignancy that is frequently fatal. It ranks as the seventh most common cancer worldwide (1). Surgery is widely accepted as the mainstay of treatment for resectable esophageal carcinoma. However, traditional open esophagectomy is associated with high morbidity and mortality (2,3). Minimally invasive esophagectomy (MIE) was recently introduced with the aim of decreasing postoperative complications and surgery-related death and improving patients’ quality of life. One multicenter randomized controlled trial performed in the Netherlands (4) demonstrated that MIE was associated with obviously decreased rates of pulmonary complications, which are usually regarded as an important risk factor for postoperative death. A modest improvement in perioperative outcomes was also found in a number of retrospective studies and meta-analyses (5-8).
Our previous study confirmed that MIE allowed for a higher number of lymph nodes to be dissected and that the surgical outcomes of MIE were at least equivalent to those of open surgery when treating patients with early-stage (stage Tis, T1, and T2) esophageal carcinoma (9). On account of the large tumor size and high rate of lymph node metastasis, whether patients with advanced esophageal carcinoma benefit from MIE in terms of perioperative outcomes and long-term survival has not been demonstrated.
In this study, we retrospectively reviewed data from our institution to determine whether patients with locally advanced stage pathologic T3 (pT3) esophageal carcinoma benefit from MIE.
Methods
Patients
A retrospective data analysis was undertaken on all patients who had undergone radical esophagectomy at Shanghai Chest Hospital from January 2013 to June 2015. A total of 229 (8%) patients with pT3 stage were enrolled in this study. Clinical data were collected from the esophageal surgery departmental database, which was established in 2010. The patients selection for the MIE or open surgery depends on the surgeon’s inclination or patients’ will (this is involved by the economic status). No matter MIE or open surgery, the surgical approach is same, right approach and at-least 2-field lymph nodes dissection were mandatory. The MIE program was first introduced in our institution at 2011, but the surgical procedure was significantly increased in the year of 2013. The inclusion criteria were as follows: the patient was diagnosed with esophageal squamous cell carcinoma (ESCC), treatment involved either open surgery or MIE, the surgical approach used was McKeown, and the pathological stage was confirmed to be T3. The patients were followed until death or June 2016. A further selection was carried out according to the 1:1 ratio propensity score matching method, a method with eight covariates (age, gender, body mass index, American Society of Anaesthesiologists score, tumor location, clinical stage, neoadjuvant therapy and pathological stage). Approval for the study was obtained from the Ethics Committee of Shanghai Chest Hospital [ID of the ethic approval: KS (P)1710]. Written informed consent was obtained from each patient or her legal representative.
Preoperative evaluation
All patients underwent upper endoscopy and were given a pathological diagnosis. Clinical staging was based on the findings of imaging examinations including enhanced computed tomography (CT) of the chest and abdomen, endoscopic ultrasonography, and radiolabeled fluorodeoxyglucose 18F whole-body positron emission tomography-computed tomography, which was performed to confirm the presence or absence of distal metastasis. Cranial magnetic resonance imaging was performed selectively.
Surgical techniques
Two-field (thoracic and abdominal) lymph node dissection and a right thoracic approach were mandatory in both the MIE and open surgery groups. The McKeown approach was used in the two groups.
In our institution, the MIE approach starts with thoracoscopic esophageal mobilization and dissection of lymph nodes in the thoracic cavity via the right side of the chest. Patients are then placed in the supine position to perform the laparoscopic procedure with gastric mobilization and upper abdominal lymph node dissection, followed by reconstruction of the neo-esophagus and performance of neck anastomosis.
In our institution, the dissected lymph nodes were calculated by the pathologist and lymph nodes in the resection specimen. The diameter of the lymph node over 5 mm will be calculated. The lymph nodes sampling along the recurrent laryngeal nerve (RLN) were defined as the lymph node in the resection specimen can be detected by the pathologist. The patients were staged according to the 6th edition of the Union for International Cancer Control esophageal cancer staging system.
Complications
Postoperative complications were defined as follows:
- Heart failure: symptoms as dyspnea and body mass increase within a short time, N-terminal pro-brain natriuretic peptide (NT-proBNP) measurement results were >450 ng/L, >900 ng/L, >1,800 ng/L for patients under the age of 50, 50–75 and over 75;
- Anastomotic leak: detection of saliva, ingested material, gastric secretions, or bile in the drain or wound. Radiographic confirmation was not required;
- Respiratory failure: reintubation or tracheostomy for weaning failure;
- Vocal cord paralysis: laryngoscopy confirmation required;
- Wound infection: local findings of erythema, drainage, subcutaneous emphysema, or tenderness requiring wound opening with positive wound culture;
- Empyema: thoracentesis was performed with positive bacterial culture. Radiographic confirmation was required;
- Chylothorax: pleural fluid with milky appearance and pleural fluid triglyceride levels >1.24 mmol/L (110 mg/dL) with a cholesterol <5.18 mmol/L (200 mg/dL);
- Deep vein thrombosis: ultrasound confirmation required;
- Pulmonary embolus: CT pulmonary angiography (CTPA) confirmation required;
- Arrhythmia: electrocardiographic confirmation required;
- GI bleeding: selective angiography and endoscopy was applicated;
- Pneumonia: radiographic confirmation with positive respiratory tract culture;
- Delirium: transient confusion confirmed by disturbances in consciousness, cognition, and perception.
Postoperative care and follow-up
Patients were transferred to the intensive care unit (ICU) in 2–3 h after the operation and extubation was performed after assessment of the patients’ respiratory function. In our institution, patients in the ICU who underwent esophagectomy would be transferred to the general ward after the patient could cough autonomously and no early anastomotic leakage was found. The nasogastric tube was removed when a esophagography was successfully performed on the 7th postoperative day. Then, patients were encouraged to sip water and the oral intake were gradually increased until the patient could tolerate soft diet without parenteral nutrition. The follow-up was performed at the 1st and 6th months and every year postoperatively with the help of examination of chest and abdomen CT scan.
Statistical analysis
Overall and recurrence-free survival curves were calculated using the Kaplan-Meier method, and comparisons were analyzed by the log-rank test. Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY). The mean, median, and standard deviation were calculated for continuous variables. Student’s t-test, the Chi-square test, and Fisher’s exact test were used to compare categorical variables between the two groups. A P value of <0.05 was considered statistically significant.
Results
In total, 229 patients were included in this study; 163 underwent open surgery and 66 underwent MIE. The median age of the patients who underwent MIE and open surgery was 61 and 62 years, respectively. Most of the patients in the MIE and open surgery groups were male (83.3% vs. 88.3%, P=0.39). There was no significant difference in age, sex, body mass index, American Society of Anesthesiologists classification between the two groups. No statistical difference was noted between MIE and open surgery in terms of clinical T staging (P=0.35) or clinical N staging (P=0.45). Twenty patients in the open surgery group received neoadjuvant chemotherapy, while none accepted neoadjuvant treatment in the MIE group. The clinical and pathologic characteristics were comparable (P>0.01) after propensity score matching (Table 1).
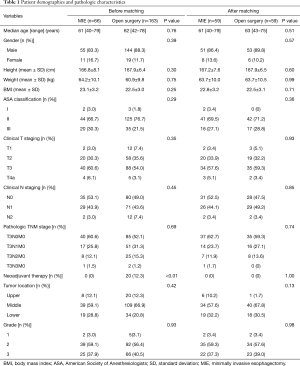
Full table
The mean operative duration was longer in the MIE (266.5±52.5 vs. 218.1±47.4 min, P<0.01; 262.3±49.2 vs. 208.2±44.2 min, P<0.01; before and after matching respectively). There was no significant difference between the two groups in terms of blood loss and R0 resection rate (Table 2).
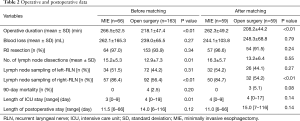
Full table
The mean number of lymph nodes dissected was 15.2±5.3 and 12.9±7.3 in the MIE and open surgery groups, respectively (P=0.01). The lymph node sampling rate of the left para-RLN was higher in the MIE than open surgery group (51.5% vs. 44.2%), but the difference was not statistically significant. However, the lymph node sampling rate of the right para-RLN was significantly different between the MIE and open surgery groups (86.4% vs. 56.4%, P<0.01).
No 90-day mortality occurred in the MIE group, but 4 (2.5%) patients died in the open surgery group. Of these four patients, two died of anastomotic leakage and multiorgan failure, one died of heart failure, and one died of pulmonary embolism and acute respiratory distress syndrome. The length of the postoperative hospital stay was not significantly different between the 2 groups, but patients in the MIE group had shorter ICU stay (Table 2).
The major complications are shown in Table 3. There was no significant difference between the two groups in overall complication rate (41.7% in open surgery group vs. 37.9% in MIE group, P=0.59). Pneumonia was the most common complication in both groups. Chylothorax developed in two patients in the open surgery group and resolved with conservative treatment.
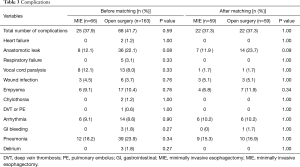
Full table
The median follow-up duration was 21.2 months (range, 12.2–41.2 months) in the open surgery group and 19.3 months (range, 12.6–40.4 months) in the MIE group. Eighteen (27.3%) patients in the MIE group and 60 (36.8%) patients in the open surgery group developed recurrence, and the predominant recurrence pattern was distant recurrence. The distribution of recurrence, whether local, regional, distant, or concurrent locoregional + distant, was not significantly different between the MIE and open surgery groups (1.5%, 12.1%, 13.6%, and 0.0% vs. 0.6%, 14.1%, 20.2%, and 1.8%, respectively; P=0.59) (Table 4). Table 4 lists the regional and distant recurrence sites; there was no significant difference between the two groups. The lung was the most frequent site of distant recurrence in both the MIE and open surgery groups (2.5% vs. 9.8%, P=0.36).
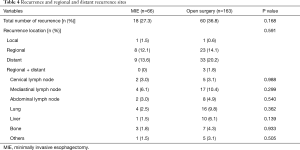
Full table
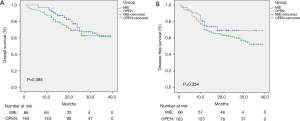
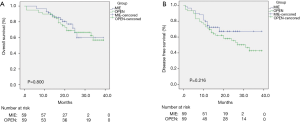
Figures 1,2 show the results of the Kaplan-Meier survival plot. The estimated overall survival (OS) rate at 2 years was 72.8% in the MIE group and 69.4% in the open surgery group (Figure 1A). The disease-free survival (DFS) rate at 2 years was 69.4% and 57.2%, respectively (Figure 1B). No statistically difference was noted in terms of OS or DFS after propensity score matching (Figure 2).
Discussion
Since Cuschieri et al. (10) and DePaula et al. (11) first introduced MIE, a number of reports have demonstrated that MIE is safe and feasible for patients with esophageal cancer in terms of short-term outcomes (3-7). However, many of these studies had selection bias because they included more patients with early-stage cancer and fewer with locally advanced stage T3 cancer, and the ratio of patients with stage T3 cancer in the MIE group ranged from 8.9% to 35.1% (3,9,12). Many institutions have not established any MIE surgical criteria for patients with stage T3 cancer, resulting in large tumor loads that demand more rigid resection of the primary tumor and extensive lymph node dissection. To the best of our knowledge, this is the first study to compare MIE and traditional open esophagectomy in patients with stage T3 esophageal carcinoma. The present study showed that MIE was associated with a higher number of dissected lymph nodes and comparable perioperative outcomes and mid-term survival rates when compared with open surgery.
Many studies have demonstrated that MIE allows for more extensive lymph node dissection (13-15). In a retrospective study, Berger et al. (16) showed that significantly more lymph nodes were dissected in the MIE than open group (20 vs. 9, respectively; P<0.01). This advantage was also shown in the area of the bilateral RLNs, especially with respect to the node sampling rate. In this study, we attribute the higher number of dissected lymph nodes to better visualization and the use of endoscopic instrumentation, which affords operators flexibility when removing the lymph nodes in the region of the bilateral RLNs. However, bias in patient selection still occurs in clinical practice. In this study, the rates of lymph node metastasis and positivity were both higher in the open surgery group; consequently, more patients in this group accepted inductive chemotherapy. A recent study showed that both open surgery and MIE approaches appear equivalent with regard to perioperative oncologic outcomes after neoadjuvant therapy (17), but this evidence was mostly derived from patients with adenocarcinoma; only a few patients had squamous cell malignancies. A comparative study is needed to clarify the influence of induction chemoradiation therapy on the outcome of MIE. In our study, the oncologic outcomes of the MIE group were at least equivalent to those of the open surgery group. For patients with stagepT3 cancer, MIE allowed for a higher number of dissected lymph nodes than did open surgery. The complete pathologic response was similar between the two groups.
In this presenting study, the estimated blood loss was higher in the MIE group (262.1±165.3 vs. 239.0±65.5, P=0.27). The two patients in the MIE group with blood loss more than 1,000 mL may be the main reason for this result, while the blood loss in the other 227 patients were no more than 450 mL. The median ICU stay were 3 days in MIE group and 4 days in open surgery group, and the recent clinical study of MIE and open surgery made by Biere et al. showed the results of 1-day ICU stay (4). The different criteria of ICU transfer may be the main reason for this result. In our institution, patients in the ICU who underwent esophagectomy would be transferred to the general ward after the patient could cough autonomously and no early anastomotic leakage was found.
One of the most important reasons for the popularization of MIE is that this procedure helps to reduce postoperative complications (18). In this study, the overall rate of major complications was lower in the MIE than open surgery group (37.9% vs. 41.7%, respectively), although there was no statistically significant difference. The most common complication in both groups was pneumonia. The patients in the MIE group showed a lower rate of pneumonia than those in the open surgery group, although the difference was not significant (18.2% vs. 23.9%, P=0.34). In a group of 80 patients undergoing MIE and open surgery, Parameswaran et al. (15) demonstrated that there was no difference in the rate of total complications between the two groups, while the rate of pulmonary complications was lower in the MIE than open surgery group (8% vs. 23%). In the present study, there was no difference in the pneumonia rate between the two groups. One of the reasons may be that stage T3 tumors are large and have invaded the outer membrane of the esophagus, resulting in difficult manipulation under thoracoscopy and a longer operating time, inevitably compromising the pulmonary tissue. Single-lung positive-pressure ventilation with artificial pneumothorax in the right thoracic cavity may also damage the pulmonary function.
Recurrence is a major factor affecting long-term survival. Extensive lymph node dissection can significantly decrease the local recurrence of ESCC. Good outcomes of node dissection in association with MIE were shown in both our previous studies and the current study (9,14). Palazzo et al. (13) compared MIE and open surgery in a series of 172 patients and showed that the recurrence rate was lower in the MIE than open surgery group (20.2% vs. 29.4%, respectively). Another retrospective study by Kauppi et al. (19) also showed that MIE had a lower recurrence rate than open surgery (34% vs. 44%, respectively). However, there was no significant difference in either of the two studies. In our study, the MIE group had lower recurrence than the open surgery group, but the difference was not significant. There were also no differences in the sites of regional recurrence between the two groups, which confirmed our conclusion that MIE is at least equivalent to open surgery in terms of oncologic outcomes. Additionally, during the mid-term follow-up, distant metastasis was the most common recurrence pattern. This recurrence pattern also indicted reliable lymph node dissection and local disease treatment via the MIE procedure.
Satomo et al. (20) performed a multivariable analysis of factors linked to postoperative survival of patients with esophageal carcinoma and concluded that the tumor stage was the one independent factor for survival. The more patients with early-stage cancer are included in such studies, the better the long-term survival will be. Consequently, their study demonstrated uncertainty of MIE in treating advanced pT3 esophageal carcinoma. Hsu et al. (21) reported that the 3-year DFS rate was better in the MIE group when treating esophageal squamous cell carcinoma (P=0.007). However, after subgroup analysis, there was no significant difference between MIE and open surgery. The 2-year OS and DFS rates in our study showed no difference between the two groups. However, more time is needed to compare the long-term survival rates between MIE and open surgery. This study has some limitations. First, this was a retrospective nonrandomized study from a single institution, and selective bias was therefore inevitable. Additionally, selection of the surgical approach was affected by the patients’ will and financial situation. Second, the rate of inductive chemotherapy was low in our study; the open surgery group contained only 12.3% of the patients. Larger multicenter, prospective, and randomized controlled studies are needed to verify these results.
In conclusions, our results show that MIE is safe and feasible in treating locally advanced stage T3 ESCC. MIE favors a high rate of lymph node dissection. The recurrence and 3-year OS rates were similar between MIE and open surgery.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81372472).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval for the study was obtained from the Ethics Committee of Shanghai Chest Hospital [ID of the ethic approval: KS(P)1710]. Written informed consent was obtained from each patient or her legal representative.
References
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790-801. [Crossref] [PubMed]
- Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003;4:481-8. [Crossref] [PubMed]
- Burdall OC, Boddy AP, Fullick J. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc 2015;29:431-7. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Zingg U, McQuinn A, DiValentino D, et al. Minimally invasive versus open esophagectomy for patients with esophageal cancer. Ann Thorac Surg 2009;87:911-9. [Crossref] [PubMed]
- Singh RK, Pham TH, Diggs BS, et al. Minimally invasive esophagectomy provides equivalent oncologic outcomes to open esophagectomy for locally advanced (stage II or III) esophageal carcinoma. Arch Surg 2011;146:711-4. [Crossref] [PubMed]
- Pham TH, Perry KA, Dolan JP, et al. Comparison of perioperative outcomes after combined thoracoscopic-laparoscopic esophagectomy and open Ivor-Lewis esophagectomy. Am J Surg 2010;199:594-8. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Ye B, Zhong CX, Yang Y, et al. Lymph node dissection in esophageal carcinoma: Minimally invasive esophagectomy vs open surgery. World J Gastroenterol 2016;22:4750-6. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- DePaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. [Crossref] [PubMed]
- Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. [Crossref] [PubMed]
- Fabian T, Martin JT, McKelvey AA, et al. Minimally invasive esophagectomy: a teaching hospital's first year experience. Dis Esophagus 2008;21:220-5. [Crossref] [PubMed]
- Parameswaran R, Veeramootoo D, Krishnadas R, et al. Comparative experience of open and minimally invasive esophagogastric resection. World J Surg 2009;33:1868-75. [Crossref] [PubMed]
- Berger AC, Bloomenthal A, Weksler B, et al. Oncologic efficacy is not compromised, and may be improved with minimally invasive esophagectomy. J Am Coll Surg 2011;212:560-6; discussion 566-8. [Crossref] [PubMed]
- Tapias LF, Mathisen DJ, Wright CD, et al. Outcomes With Open and Minimally Invasive Ivor Lewis Esophagectomy After Neoadjuvant Therapy. Ann Thorac Surg 2016;101:1097-103. [Crossref] [PubMed]
- Dolan JP, Kaur T, Diggs BS, et al. Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surg Endosc 2013;27:4094-103. [Crossref] [PubMed]
- Kauppi J, Räsänen J, Sihvo E, et al. Open versus minimally invasive esophagectomy: clinical outcomes for locally advanced esophageal adenocarcinoma. Surg Endosc 2015;29:2614-9. [Crossref] [PubMed]
- Satomoto M, Suzuki A, Uchida T, et al. Potential influence of pre and intraoperative factors on postoperative recurrence and survival in patients undergoing radical resection of esophageal cancer. Masui 2014;63:1344-9. [PubMed]
- Hsu PK, Huang CS, Wu YC, et al. Open versus thoracoscopic esophagectomy in patients with esophageal squamous cell carcinoma. World J Surg 2014;38:402-9. [Crossref] [PubMed]


