Is compensatory hyperhidrosis after thoracic sympathicotomy in palmar hyperhidrosis patients related to the excitability of thoracic sympathetic ganglions?
Introduction
Primary palmar hyperhidrosis is characterized by oversecretion of the eccrine sweat glands in the hands due to unknown etiology (1). Most researchers believe that hyperhidrosis is associated with dysfunction of the sympathetic nervous system (2,3). Dai et al. (4) found that choline acetyltransferase (ChAT) and vasoactive intestinal peptide (VIP) expression in sympathetic ganglions was significantly increased in palmar hyperhidrosis patients compared with individuals who did not have hyperhidrosis. Therefore, the authors considered thoracic sympathetic ganglion over-activity as a possible pathogenic mechanism of palmar hyperhidrosis. Thoracic sympathicotomy is the only effective and radical treatment for primary palmar hyperhidrosis (5). Compensatory hyperhidrosis is one of the most common postoperative complications and causes some patients to regret undergoing surgery due to severe sweating in other parts of the body. The pathogenic mechanism of compensatory hyperhidrosis is extremely complex. Lin et al. (6) hypothesized that the reflex arc from the sympathetic nervous system to the hypothalamus is destroyed by surgery, which leads to dysfunctional sweating control in other parts of the body. However, no reports have examined whether compensatory hyperhidrosis is associated with thoracic sympathetic ganglion excitability. Hence, this study was designed to explain the relationship between compensatory hyperhidrosis and thoracic sympathetic ganglion excitability by evaluating ChAT, VIP, and synaptophysin expression in the thoracic sympathetic ganglions of patients with compensatory hyperhidrosis and to evaluate the effectiveness of thoracoscopic T4 sympathicotomy for the treatment of palmar hyperhidrosis.
Methods
Clinical data
From January 2013 to December 2016, thoracoscopic T4 sympathectomies were performed in 66 patients to treat primary palmar hyperhidrosis. The patient cohort included 35 males and 31 females with a mean age of 22.9±4.1 years (range, 15–37 years). Each patient’s degree of hyperhidrosis was classified according to the Lin-Telaranta criteria (1). There were 6 cases of moderate hyperhidrosis and 60 cases of severe hyperhidrosis but none of mild hyperhidrosis. Hyperhidrosis occurred in the face in 4 patients, the axilla in 25 and the pedal in 56. All patients were diagnosed with primary palmar hyperhidrosis excluding secondary hyperhidrosis caused by hyperthyroidism, diabetes, or spinal cord injury. Thoracoscopic bilateral T4 sympathectomies were successfully performed in all patients. T4 sympathetic ganglions were collected prospectively. The specimens were soaked in formalin immediately. Written informed consent was acquired from each patient prior to publishing this report and collecting the specimens. Our study obtained ethics approval from Medical Ethics Committee of Xiamen Hospital of Traditional Chinese Medicine and the approval number was 2013 (3).
Operative methods
General anesthesia using a laryngeal mask or single-lumen endotracheal tube with a low tidal volume was applied in all cases. The patients were then placed in semi-fowler’s position with both arms abduced to 90°. One port for the instrument was made in the anterior axillary line at the level of the third intercostal space, and another port for the telescope was created in the mid-axillary line at the level of the fourth intercostal space. A 5-mm, 30° telescope was inserted first to explore the thoracic cavity. After pleural adhesion was excluded, CO2 gas was installed into the thoracic cavity to maintain the intrathoracic pressure between 4 to 6 mmHg. After the lung was atrophic, the thoracic sympathetic chain was revealed clearly beside the spine. The T4 sympathetic ganglion was located, and the sympathetic chain was then cut using electrocautery on the surface of the fourth capitulum costae. The T4 sympathetic ganglion was removed with graspers and immediately soaked in formalin. To remove the potential bypass nerve fibers, the transection range was extended approximately 2 cm laterally along the fourth capitulum costae. When the hand temperature increased, the operation was considered successfully completed. The surgical procedures were conducted first on the right side first and then the left in an identical manner.
Experimental methods
- Reagents: RabMAb-anti-choline acetyltransferase antibody, anti-VIP antibody, RabMAb-anti-synaptophysin antibody, polymerase combination antibody (anti-mouse/IgG), diaminobenzidine (DAB) kit, and phosphate buffered saline.
- Instruments: LEICA RM2245 microtome, OLYMPUS BX43F microscope, SAKURA Tissue-Tek TEC 5 Embedder, SAKURA Tissue-Tek prisma dyeing machine, and Roche BenchMark GX immunohistochemistry machine.
The expression of ChAT, VIP, and synaptophysin in thoracic sympathetic ganglions
The specimens were sliced after paraffin embedding. Immunohistochemistry methods were used to detect the expression of ChAT, VIP, and synaptophysin. The expression quantity was assessed using a semi-quantitative method: “−” indicated that a sample was negative, “+” indicated positivity from 1% to 25%, “++” indicated positivity from 26% to 50%, “+++” indicated positivity from 51% to 75%, and “++++” indicated positivity from 76% to 100%
Follow-up
Follow-up was conducted for all patients by telephone, e-mail, letter, or outpatient service. A questionnaire was created to record data about compensatory hyperhidrosis and classify it as mild, moderate, or severe (7). The severity of compensatory hyperhidrosis was assessed by particular conditions, such as whether patients could quietly remain in a room with a proper temperature that was not hot and felt calm. The postoperative remission rate was defined as the number of cases in which postoperative hyperhidrosis disappeared or decreased divided by the number of preoperative hyperhidrosis cases.
Statistical analysis
The data were analyzed using SPSS 19.0 for Windows. Categorical variables were analyzed using the Chi-square or Fisher’s exact tests, and continuous data were analyzed using the unpaired Student’s t-test. The Mann-Whitney U test was used to analyze the immunohistochemistry results. P values of less than 0.05 were considered to be statistically significant.
Results
Clinical data of palmar hyperhidrosis patients
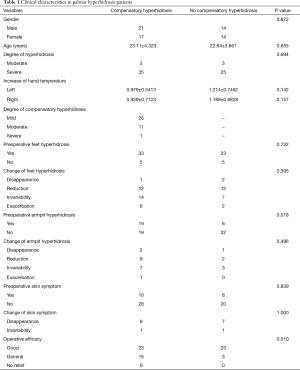
The median follow-up period was 15 months (range, 5 to 31 months). Among the 66 patients, 38 cases (57.6%) of compensatory hyperhidrosis were observed. The clinical data are shown in Table 1. Twenty-six patients suffered from mild compensatory hyperhidrosis, which had little effect on daily life, 11 cases were moderate, and 1 case was severe. Patients in the compensatory hyperhidrosis group were significantly more likely to experience combined axilla hyperhidrosis (76.0% vs. 46.3%, respectively, P=0.018). With this exception, the clinical data were similar between the compensatory hyperhidrosis group and the no compensatory hyperhidrosis group. Palmar hyperhidrosis was resolved in all patients, and the overall satisfaction rate was 98.5% (65/66). The remission rate was 48.2% (27/56) in pedal hyperhidrosis, 56.0% (14/25) in axilla hyperhidrosis and 88.9% (16/18) in skin symptoms. No chapped palm skin was found, but some patients experienced mildly wet palms under conditions of high temperature or nervousness. Palmar hyperhidrosis did not recur in any patient.
Full table
ChAT expression in T4 sympathetic ganglions
ChAT was expressed in both the cytoplasm and nucleus. The level of ChAT expression was not significantly different between the moderate and severe hyperhidrosis patients (u=−0.817, P=0.414). As shown in Table 2, ChAT expression was not significantly different between the compensatory hyperhidrosis group and the no compensatory hyperhidrosis group (P=0.356). Further stratified analysis showed that ChAT expression in the mild group was similar to that in the moderate/intense group (P=0.089).

Full table
VIP expression of T4 sympathetic ganglions
VIP expression was detected in cytoplasm and was similar between the cases of moderate and severe hyperhidrosis (u=−0.600, P=0.548). Table 3 shows that no differences in VIP expression were observed between the compensatory hyperhidrosis group and the control group (P=0.071). In addition, the VIP expression in the mild group did not differ from that in the moderate/intense group (P=0.124).

Full table
Synaptophysin expression in T4 sympathetic ganglions
Synaptophysin expression was also discovered in cytoplasm but was not significantly different between the moderate and severe hyperhidrosis groups (u=−0.605, P=0.545). Table 4 shows that the synaptophysin expression levels were not significantly different between the compensatory hyperhidrosis group and the control group (P=0.141) as well as between the mild group and the moderate/intense group (P=0.149).

Full table
Discussion
At present, thoracoscopic thoracic sympathicotomy is the preferred treatment for palmar hyperhidrosis due to several advantages, including minimal invasion, significant efficacy, and rapid recovery (5,8). Compensatory hyperhidrosis is the most common postoperative complication (1,9) and is also one of the main factors that prevents widespread application of this technique. Compensatory hyperhidrosis refers to a postoperative increase of sweating in regions of the body where sympathetic innervation was not removed, such as the chest, abdomen, back, hip, thigh, or shank (10). As reported in the literature, the compensatory hyperhidrosis rate varies from 4.2% to 96.4% due to different extents of sympathicotomy and different definitions of compensatory hyperhidrosis (11). A previous study confirmed that reducing the extent of sympathicotomy or lower the operation level decreases the incidence of compensatory hyperhidrosis (12,13). However, as shown in Table 1, even though we only resected the thoracic sympathetic chain at the T4 level, the compensatory hyperhidrosis rate reached 57.6%. Except in mild patients, 18.2% of patients were affected by moderate or severe sweating. The mechanism of compensatory hyperhidrosis is unclear and complicated, which is also the present focus of the research.
A study conducted by Dai et al. (4) revealed that the activity of T3 and T4 sympathetic ganglions were higher in palmar hyperhidrosis patients than in individuals without hyperhidrosis; for palmar hyperhidrosis patients, the activity of the T3 sympathetic ganglion was greater than that of T4. It has been reported that the incidence of compensatory hyperhidrosis is higher after T3 sympathicotomy than T4 sympathicotomy (13,14). Presumably, the higher the activity of the thoracic sympathetic ganglion, the more the negative feedback signals from the sympathetic nerve to the hypothalamus are blocked and compensatory hyperhidrosis occurs. Therefore, the purpose of this paper was to explore the expression levels of ChAT, VIP, and synaptophysin in thoracic sympathetic ganglions to reveal the relationship between thoracic sympathetic ganglion excitability and compensatory hyperhidrosis. ChAT is the rate-limiting enzyme for the synthesis of acetylcholine, and detecting ChAT expression can indirectly reflect the status of the cholinergic nerve system (15-17). VIP is a type of peptide hormone that exists in the sympathetic nerve fibers and can stimulate sweat gland secretion by way of cyclic adenosine monophosphate (cAMP). In addition, VIP increases sweat gland secretion by selectively enhancing the combination of acetylcholine and M-type receptor and expanding the peripheral vasculature to increase blood flow (18,19). Synaptophysin expression can reflect the strength of the nerve impulse transmission because it is able to quickly adjust neurotransmitter release (20-22). Hence, quantitative detection of the ChAT, VIP, and synaptophysin expression levels was used to reflect sympathetic nerve excitability in this study. The results showed that ChAT, VIP, and synaptophysin expression levels were not significantly different, not only between the compensatory hyperhidrosis groups and the control group but also between the mild group and the moderate/severe group. Consequently, we did not observe a relationship between compensatory hyperhidrosis and sympathetic nerve excitability. Compensatory hyperhidrosis may occur through another unknown mechanism. However, the rate of compensatory hyperhidrosis was greater in patients with axilla hyperhidrosis than without. Further studies may reveal other potential mechanisms of compensatory hyperhidrosis.
As shown in the results, the incidence of moderate/severe compensatory hyperhidrosis was only 18.2%, and only one severe case was found, which is similar to results from previous studies (13,14,23). The remission rate of palmar hyperhidrosis was 100%. Additionally, 48.2% of pedal hyperhidrosis, 56.0% of axilla hyperhidrosis, and 88.9% of skin symptoms were relieved. No chapped palm skin was found postoperatively, and 98.5% of patients were satisfied with the surgical effects. Thus, thoracoscopic T4 sympathicotomy is the ideal technique for palmar hyperhidrosis due to its satisfactory efficacy and low incidence of compensatory hyperhidrosis and chapped skin.
Although there was no significant correlation between compensatory hyperhidrosis and thoracic sympathetic ganglion excitability, this research is limited to a small sample, single center, and short follow-up time. We will conduct ongoing follow-up to track changes in compensatory hyperhidrosis. Compensatory hyperhidrosis is complex and lacks a therapeutic treatment; therefore, further studies should be performed to uncover mechanisms and seek effective intervention for compensatory hyperhidrosis.
Acknowledgements
Funding: The study was sponsored by the Projects of Fujian Province Health Department for Youth (2013-2-95) and the Guidance Project of Science and Technology Plan of Xiamen in 2014 (3502Z20149010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study obtained ethics approval from Medical Ethics Committee of Xiamen Hospital of Traditional Chinese Medicine and the approval number was 2013.
References
- Lai YT, Yang LH, Chio CC, et al. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery 1997;41:110-3; discussion 113-5. [Crossref] [PubMed]
- Atkins JL, Butler PE. Hyperhidrosis: a review of current management. Plast Reconstr Surg 2002;110:222-8. [Crossref] [PubMed]
- Alric P, Branchereau P, Berthet JP, et al. Video-assisted thoracoscopic sympathectomy for palmar hyperhidrosis: results in 102 cases. Ann Vasc Surg 2002;16:708-13. [Crossref] [PubMed]
- Dai ZJ, Tu YR, Li X, et al. Expression and significance of choline acetyltransferase and vasoactive intestinal peptide in thoracic sympathetic ganglion of patients with palmar hyperhidrosis. Chin J Exp Surg 2007;24:1017-8.
- Cerfolio RJ, De Campos JRM, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Lin CC, Telaranta T. Lin-Telaranta classification: the importance of different procedures for different indications in sympathetic surgery. Ann Chir Gynaecol 2001;90:161-6. [PubMed]
- Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg 2004;78:427-31. [Crossref] [PubMed]
- Bonjer HJ, Hamming JF, du Bois NAJJ, et al. Advantages of limited thoracoscopic sympathectomy. Surg Endosc 1996;10:721-3. [Crossref] [PubMed]
- Wilson MJ, Magee TR, Galland RB, et al. Results of thoracoscopic sympathectomy for the treatment of axillary and palmar hyperhidrosis with respect to compensatory hyperhidrosis and dry hands. Surg Endosc 2005;19:254-6. [Crossref] [PubMed]
- Shelley WB, Florence R. Compensatory hyperhidrosis after sympathectomy. N Engl J Med 1960;263:1056-8. [Crossref]
- Kopelman D, Hashmonai M. The correlation between the method of sympathetic ablation for palmar hyperhidrosis and the occurrence of compensatory hyperhidrosis: a review. World J Surg 2008;32:2343-56. [Crossref] [PubMed]
- Li X, Tu YR, Lin M, et al. Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg 2008;85:1747-51. [Crossref] [PubMed]
- Liu Y, Yang J, Liu J, et al. Surgical treatment of primary palmar hyperhidrosis: a prospective randomized study comparing T3 and T4 sympathicotomy. European Journal of Cardio-Thoracic Surgery 2009;35:398-402. [Crossref] [PubMed]
- Wolosker N, Yazbek G, Ishy A, et al. Is sympathectomy at T4 level better than at T3 level for treating palmar hyperhidrosis? J Laparoendosc Adv Surg Tech A 2008;18:102-6. [Crossref] [PubMed]
- Gauda EB, Cooper R, Johnson SM, et al. Autonomic microganglion cells: a source of acetylcholine in the rat carotid body. J Appl Physiol (1985) 2004;96:384-91. [PubMed]
- Bakhit C, Armanini M, Wong WL, et al. Increase in nerve growth factor-like immunoreactivity and decrease in choline acetyltransferase following contusive spinal cord injury. Brain Res 1991;554:264-71. [Crossref] [PubMed]
- Weihe E, Schäfer MK-H, Schütz B, et al. From the cholinergic gene locus to the cholinergic neuron. J Physiol Paris 1998;92:385-8. [Crossref] [PubMed]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science 1970;169:1217-8. [Crossref] [PubMed]
- Mo N, Dun N. Vasoactive intestinal polypeptide facilitates muscarinic transmission in mammalian sympathetic ganglia. Neurosci Lett 1984;52:19-23. [Crossref] [PubMed]
- Calhoun ME, Jucker M, Martin LJ, et al. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol 1996;25:821-8. [Crossref] [PubMed]
- Calhoun ME, Kurth D, Phinney AL, et al. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging 1998;19:599-606. [Crossref] [PubMed]
- Alder J, Lu B, Valtorta F, et al. Calcium-dependent transmitter secretion reconstituted in Xenopus oocytes: requirement for synaptophysin. Science 1992;257:657-61. [Crossref] [PubMed]
- Choi BC, Lee YC, Sim SB. Treatment of palmar hyperhidrosis by endoscopic clipping of the upper part of the T4 sympathetic ganglion. Preliminary results. Clin Auton Res 2003;13 Suppl 1:I48-51. [Crossref] [PubMed]


