Effectiveness and safety of simultaneous hybrid thoracoscopic endocardial catheter ablation of atrial fibrillation in obese and non-obese patients
Introduction
The prevalence of atrial fibrillation (AF) is increasing, with projected prevalence rates of 12 to 15 million individuals in the USA (1,2). When coupled with the obesity epidemic (1), the two diseases often occur together. The body mass index (BMI) is a commonly used parameter used to define obesity (BMI ≥ 30), with increasing evidence demonstrating significant associations between obesity and AF (3,4). One systematic review suggested that obesity increased risk of AF by 49% over the general population (5), whilst another meta-analysis estimates 3.5–5.3% excess risk of AF for every unit of BMI increase (6). Given the proliferative rise in AF incidence in the setting of an obesity epidemic, there is a strong need to understand their associations and complex interplay of risk factors from a clinical perspective and particularly when considering medical, interventional and surgical therapies for AF.
Catheter ablation, primarily by pulmonary vein isolation (PVI), is an effective treatment for AF. PVI can be achieved in more than 95% of patients by the conclusion of the procedure. The advantages offered by the percutaneous catheter approach include the use of multipolar catheters and three-dimensional mapping technologies, which allows identification of the nature of the atrial substrate and allows for customizable lesion sets for ablations. Approximately three quarters of all patients achieve freedom from AF when treated with ablation (7-9). Although promising, these results are suboptimal, particularly in patients with persistent and long-standing persistent AF (10-12), which is partly due to the lack of transmurality in some lesions as well as gaps in ablation. In some cases of persistent or long-standing AF or AF refractory to medical and catheter ablation, surgical ablation offers an alternative option (13-18). In the FAST randomized controlled study (19), it was demonstrated that the video-assisted surgical approach may achieve superior success rates to catheter ablation in the short term, although the patient is at risk of higher procedural complications including pneumothorax, major bleeding and pacemaker requirements.
The development of new technologies and advanced techniques has seen an increase in a multidisciplinary approach in the treatment of AF involving both cardiac surgeons and electrophysiologists (20). The hybrid approach has been introduced in an attempt to improve results in catheter ablation and surgical ablation alone (21,22). This approach combines the advantages of both catheter and surgical ablation approaches, with a procedure that produces superior transmural lesions epicardially, whilst allowing endocardial identification of ablation targets to customize lesion sets and endocardial touch-ups to close conduction gaps. The procedure combines both thoracoscopic epicardial ablation with a percutaneous trans-septal procedure (23,24). The efficacy and benefits of the hybrid procedure appear promising, with reported freedom from AF rates of 78% to 100% at 6 months (25).
There have been previous reports that obesity is an independent predictor of procedural failure after catheter ablation (26). On the other hand, some studies have reported no differences in efficacy of catheter ablation in obese and non-obese patients (27). Metabolic syndrome has also been associated with higher recurrence rates after catheter ablation (28). Currently there are no studies examining the differences in efficacy of hybrid thoracoscopic epicardial ablation and catheter ablation on patients with and without obesity. To address this question, the present study aimed to determine if there were any differences in freedom from AF success rates in obese versus non-obese patients undergoing a hybrid ablation procedure.
Methods
Study population
For our analysis, all consecutive patients from January 2010 to January 2015 undergoing PVI and posterior left atrial wall isolation with linear lesions by hybrid thoracoscopic ablation for symptomatic persistent, long-standing persistent and paroxysmal AF in the University Hospital, Maastricht, were identified and included. As per the institutional Ethics Board, no ethical approval was required for the present study as the hybrid approach was standard of care in our institution and patients were not randomized. The population comprised 10 obese patients (BMI ≥ 30) and 51 non-obese (BMI <30) patients.
In our centre, the indications to hybrid thoracoscopic ablation are given to patients with persistent, long-standing persistent or paroxysmal AF and severe atrial dilation. Definitions of persistent, long-standing persistent and paroxysmal AF, success and failure, and complications were based on the 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of AF (10). Exclusion criteria included presence of intracavitary thrombus, uncontrolled heart failure, presence of severe coronary artery disease, and moderate or severe valvular disease.
Pre-procedural management
The left ventricular ejection fraction, intracavitary dimensions, and presence of structural and valvular disease were assessed by transthoracic echocardiography (TTE). Transoesophageal echocardiography (TOE) was performed on the day of the procedure to exclude the presence of thrombi. Pulmonary vein anatomy was evaluated before ablation by cardiac computed tomography (CT). Oral anti-coagulation therapy (OAC) was interrupted 2 days prior and replaced by low-molecular-weight heparin (LMWH) therapy. For patients taking novel anticoagulant agents, medication was stopped as follows: (I) the last dose of rivaroxaban was given in the morning 2 days prior to ablation; and (II) the last dose of dabigatran or apixaban was given in the evening 2 days prior to ablation. All patients provided written informed consent for the ablation procedures. As per the institutional Ethics Board, no ethical approval was required for the present study.
Hybrid procedure: thoracoscopic epicardial ablation
The procedure was performed in the cardiac electrophysiology laboratory as previously described (29). All procedures were performed by the same cardiac surgeon (Mark La Meir, University Hospital, Maastricht, the Netherlands). Briefly, general anaesthesia was used with a double-lumen endobronchial tube placement for selective lung ventilation. In all patients, bilateral thoracoscopic access with 5 mm ports was used. Antral isolation of both pairs of pulmonary veins was performed with 4-6 applications using a bipolar RF clamp (AtriCure, Inc., West Chester, OH, USA). Then, a roof line (connecting both superior pulmonary veins) and an inferior line (connecting both inferior pulmonary veins) were performed epicardially using a bipolar RF pen or a linear pen device (Isolator Pen and Coolrail, AtriCure, Inc.) achieving the ‘box’ lesion for the posterior wall isolation. Furthermore, if the right atrium was dilated, two additional ablation lines were created: one encircling the superior vena cava (SVC) using the clamp and the other connecting both caval veins using the pen (bicaval line). Left atrial appendage (LAA) clipping was performed in 21 patients (34.4%; AtriClip, AtriCure, Inc.).
Hybrid procedure: percutaneous electrophysiological examination and ablation
Via the femoral approach, a decapolar coronary sinus catheter (Biosense Webster, Inc., Diamond Bar, CA, USA) was placed under fluoroscopy, and a double transseptal puncture was performed using a long 8-Fr sheath (SL0, St. Jude Medical, Minnesota, USA) under both the TOE and fluoroscopy guidance. Directly after the first transseptal puncture, patients underwent heparinization (1,000 U heparin per 10 kg body weight with subsequent additional applications) with targeted activated clotting time >300 s. A detailed electroanatomic map of the left atrium (LA) was created with a non-fluoroscopic navigation system (CARTO system; Biosense Webster, Inc.) using the circular mapping catheter (Lasso, Biosense Webster, Inc.) and the open-irrigated 3.5-mm tip RF ablation catheter (NaviStar ThermoCool; Biosense Webster, Inc.). Initially, pulmonary vein entry and exit block were confirmed along with the verification of posterior wall isolation. The entry block of the pulmonary veins and the posterior box was defined as the absence of atrial bipolar signals and was evaluated with a circular mapping catheter. Exit block was defined as local capture in the pulmonary vein or posterior wall during pacing (output 10 mA and pulse width 2 ms) without conduction to the LA. If the block was not present, additional endocardial RF ablation was performed to close the conduction gaps. A power-controlled mode with a power limit of 35 W and a maximum temperature of 48 °C was used until the block was not achieved. If the patient was known to have typical flutter or if this arrhythmia occurred during the procedure, the cavotricuspid isthmus (CTI) was ablated endocardially, and a mitral line was achieved if the patient developed mitral isthmus-dependent flutter during the procedure. Furthermore, if AF persisted, left and right complex fractionated atrial electrogram (CFAE) mapping ablation was performed. The target sites were defined as the fastest local repetitive electrical activity, multiple component fragmented signal, or activation delay between the distal and proximal bipolar electrodes covering the majority of the cycle length. The endpoint for ablation was regularization or disappearance of the local signal, conversion to sinus rhythm (SR) or to stable atrial flutter. In patients who did not convert to SR during the ablation, electrical cardioversion was performed. All ablation lines were revisited in SR to confirm bidirectional conduction block using the standard criteria.
Post-procedural management
After the procedure, patients underwent continuous telemetric monitoring until discharge from the hospital. Before discharge, TTE was performed in all patients in order to exclude post-procedural pericardial effusion. LMWH was started the same evening following the ablation, and on the third postoperative day OAC was reinitiated. Patients restarted their preoperative antiarrhythmic drug (AAD) regimes as soon as possible. Oral anticoagulation and AADs were continued for at least 3 months.
Follow-up
Clinical follow-up consisted of physical examinations, electrocardiogram and 24-h Holter recording performed every 6 months. Any symptoms following ablation were deemed as deserving Holter monitoring. A blanking period of 3 months was considered for the study. All documented episodes of atrial tachycardia lasting ≥ 30 s were considered as a recurrence.
Statistical analysis
Normal values were expressed as mean ±1 standard deviation (SD), non-normal values as median and IQR, and categorical variables as percentages. The Mantel-Haenszel Chi-square was employed to establish differences among groupings. Univariate analyses of relevant risk factors for success rates on AAD or off AAD were conducted by Chi-square or Fisher’s exact tests of categorical data and Student’s t-tests of continuous data to compare the differences between patients with freedom from AF on AAD or off AAD. Statistical analysis was performed using SPSS release 12.0 (SPSS, Chicago, IL, USA). P values less than 0.05 were considered significant.
Results
Study population
Sixty-one patients (45 males, 60±9 years) were considered for the present retrospective analysis. Ten patients were obese (BMI ≥ 30) and 51 were not obese (Table 1). The indication for the hybrid procedure was persistent AF in 25 non-obese patients and 4 obese patients (47%), long-standing persistent AF in 3 non-obese patients and no obese patients (5%) and paroxysmal AF in 23 non-obese patients and 6 obese patients (48%). There was no difference between obese versus non-obese cohorts in terms of the proportion of paroxysmal AF (60% vs. 45.1%, P=0.496), persistent AF (40% vs. 49%, P=0.735) or permanent AF (0% vs. 5.9%, P=1.00). All patients had failed ≥1 class I or III AADs.
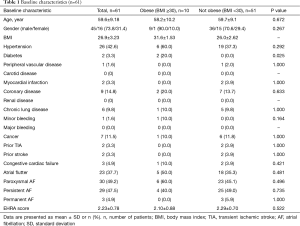
Full table
The average age of the obese and non-obese groups (58.2 vs. 59.7 years, P=0.672) were found to be similar. In terms of baseline medical comorbidities, no significant differences between the obese versus non-obese groups was found in terms of hypertension, peripheral vascular disease, carotid artery disease, coronary artery disease, renal disease, lung disease, bleeding, prior TIA or strokes, or cardiac failure. The obese group had a significantly higher proportion of patients with diabetes compared to the non-obese group (20% vs. 0%, P=0.025). With regards to the use of anti-arrhythmic medications and other medications, there was no significant difference found between the obese versus non-obese cohorts (Table S1).
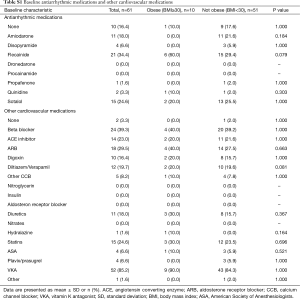
Full table
In terms of baseline ECG and echocardiographic parameters (Table S2), the mean left atrial diameter was 45±4 mm in obese patients versus 44±5 mm in non-obese patients (P=0.497). The mean left atrial volume was 91±18 cm3 in obese patients versus 93±25 cm3 in non-obese patients (P=0.812). The mean left ventricular ejection fraction was 58%±8% in obese patients versus 59%±7% in non-obese patients (P=0.768). The mean EHRA score of AF-related symptoms was 2.1±0.9 in obese patients versus 2.3±0.7 in non-obese patients (P=0.522). Overall, the mean duration of having AF was 5.2±4.6 years for the population studied. Twenty-one (34.4%) patients had a prior ablation procedure for AF prior to the present hybrid ablation.
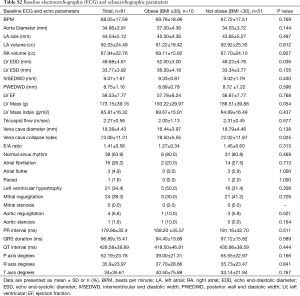
Full table
Hybrid procedure
Operative details are outlined in Table S3. There was no significant difference between the groups in terms of the ablation devices used (CryoCath catheter, AtriCure bipolar clamp, AtriCure cool rail, and AtriCure Isolator Pen). In terms of the ablation lines used, there was also no significant differences between the obese versus non-obese cohorts including roof lines, inferior lines, left isthmus, inter-caval line, IVC, SVC, and right isthmus lines. Endocardial touch-up following the primary hybrid procedure was required in 70% of the obese group and 49% of the non-obese group, but this difference was not found to be significantly different (P=0.307).
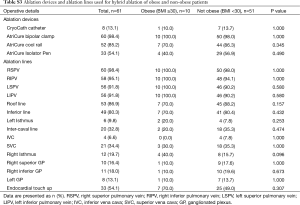
Full table
Outcomes
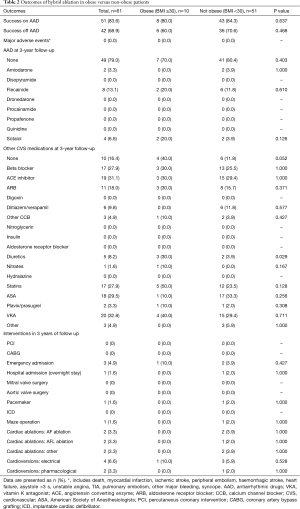
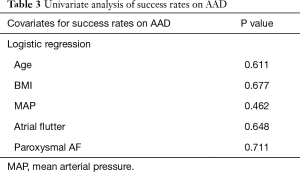
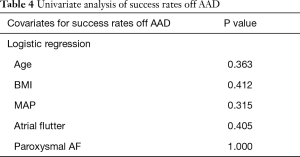
At 3-year follow-up, there was no significant difference found between success on AAD between the obese versus non-obese groups (80% vs. 86%, P=0.637) (Table 2). Furthermore, success off AAD were also found to be comparable between obese versus non-obese patients (60% vs. 70.6%, P=0.468). There were no major adverse complications in either cohort of patients. With regards to the use of AAD at 3-year follow-up, the obese group had 2 patients on flecainide and 2 patients on sotalol. The non-obese group had 2 patients on amiodarone, 6 patients on flecainide and 2 patients on sotalol. With regards to cardiovascular medications at 3-year follow-up, the only significant difference found was for diuretics, which was significantly higher in the obese group versus non-obese group (30% vs. 3.9%, P=0.029). Univariate analysis demonstrated that none of age, BMI, MAP, atrial flutter nor AF type were significantly associated with success rates on AAD (Table 3) or off AAD (Table 4).
Full table
Full table
Full table
Discussion
Our results suggest that hybrid thoracoscopic epicardial and catheter-based endocardial ablation is equally efficacious in patients with and without obesity. Although the sample size was small, we found freedom from recurrence of AF at follow-up of 60% in obese and 72% in non-obese patients. Given the mixed reports on the benefits of ablation in obese patients (26,27), our results suggest that the hybrid procedure may be a viable option in obese and non-obese patients. Further trials are required, involving larger sample sizes in order to adequately assess the efficacy and safety of this procedure in obese versus non-obese patients.
Obesity has been associated with known risk factors for AF, including inflammation, autonomic dysfunction, atrial enlargements and diastolic dysfunction (30). Obesity and AF have been increasing in epidemic proportions in the USA, with over 10 million patients in the USA affected by AF by 2050, of which 60% will be due to obesity (2). This highlights the importance of understanding how obesity can impact the outcomes of the interventional and surgical treatments for AF patients, particularly in the context of rapidly evolving techniques such as hybrid epicardial and catheter ablation.
There are numerous proposed mechanisms by which obesity is associated with AF. Obesity is known to cause structural and electrical atrial remodelling, and is associated with increasing atrial volume, fibrosis and lipidosis (31). Weight gain can also induce structural and histological changes in cardiomyocytes, which may predispose patients to chaotic rhythms such as AF (31,32). Furthermore, obesity may also be associated with pericardial fat, causing increased inflammation and local atrial infiltration, leading to increased risk of AF (30). Obesity has been found to induce impaired diastolic function, leading to atrial stretch, which can lead to greater signal complexity at the pulmonary vein-atrial junction (33). Additionally, obesity leads to increased risk of hypertension, structural heart disease, diabetes mellitus and obstructive sleep apnoea that in turn beget AF (27). Furthermore, weight fluctuations have been shown to be associated with AF recurrence (34), whilst weight increases have been associated with AF progression (35). The converse has also been shown in landmark studies demonstrating that weight loss is associated with lower recurrence of AF and increased AF-free survival following ablation (34,36). It is not unreasonable to expect similar outcomes for patients undergoing thoracoscopic surgical ablation and hybrid ablation for AF, however, there is limited evidence available for these procedures to address these specific questions.
Thoracoscopic surgical ablation is an effective method of ablation which incorporates many of the lesions in the Cox IV maze surgical procedure. There have been reports of 70% to 87% success rates in patients with paroxysmal AF (37-39) and a lower rate of 39% to 62% for persistent AF at 6 months (37). The primary rationale behind the hybrid procedure incorporating catheter ablation is the ability to identify gaps and offer additional ablation of these gaps, which can be achieved via the endocardial approach with multipolar catheters and three-dimensional mapping technologies. In this way, the surgeon uses a video-assisted thoracoscopic approach to isolate the pulmonary veins and the posterior wall of the LA epicardially whereas the electrophysiologist can evaluate the endpoints of the ablation and offer additional endocardial ablation as needed. Furthermore, there may be some lesions that are difficult to access from an epicardial surface. Finally, combining an endocardial with an epicardial approach can help ensure transmurality of lesions, particularly in areas of scarred or thick heart tissue.
Ablation in patients who have persistent or long-standing persistent AF is less effective and has poorer outcomes (40). Outcomes for catheter ablation for persistent and especially long standing persistent AF have been poor, with 1- and 5-year arrhythmia free survival rates of 35.3% and 16.8%, respectively (12). Our obese population did not have a significantly different proportion of patients with persistent AF compared to the non-obese patients (40% and 49%). Obese patients face greater risks of complications from ablation procedures due to their comorbidities. During catheter ablation, hemodynamic intolerance to general anaesthesia, increased risk of stroke and difficulty with endotracheal intubation are all concerns. Additionally, procedure times are usually longer and radiation exposure greater in obese patients (27).
The presence of pericardial fat is associated with higher rates AF, symptom burden and poorer outcomes after AF ablation (30). Pericardial fat has been linked with increased expression of local inflammatory markers, particularly in the LA, which may play a role in AF genesis (41,42). A recent study showed lower success rates after non-hybrid AF ablation in obese patients. Given hybrid ablation may a suitable alternative to repeat endocardial ablation, it is important to assess the outcomes of hybrid ablation in obesity (24).
A large prospective study comparing 485 patients with metabolic syndrome and 1,011 control patients undergoing catheter ablation showed that metabolic syndrome patients had higher rates of arrhythmia recurrence in the non-paroxysmal subgroup. However the metabolic syndrome patients had improved physical quality of life, which was not seen in the non-metabolic syndrome group (43). Reports from the AFFIRM trial on 2,492 patients, identified better outcomes, including 3-year mortality rates in obese patients compared to non-obese patients after ablation. Our data suggests obese patients may also be possible candidates for the newer hybrid procedure. However larger studies are required in order to validate the safety and efficacy of this procedure.
Limitations
The present study was constrained by several limitations. Firstly, there was a small sample size of 61 patients which may have underpowered the study. Despite this, the present study is significant because very few centers worldwide have the capabilities and facilities at the moment to perform this newly introduced hybrid ablation procedure. As such, the present study represents the early evidence available specifically for this technique. Future studies should compare larger multi-institutional cohorts of obese and non-obese patients using the hybrid procedure. Secondly, the present study only focused on patients undergoing hybrid ablation procedure with a retrospective study design, which may be susceptible to selection bias. Currently, a randomized controlled trial is underway to compare outcomes of hybrid versus catheter ablation for persistent AF (HARTCAP-AF ClinicalTrials.gov identifier NCT02441738). This may provide higher quality evidence to address the role of hybrid ablation in treatment of AF in obese and non-obese patients. A strength of the current study is the moderate and consistent follow-up results for the 61 patients at 3 years, demonstrating favourable success rates compared to published outcomes in the literature. The longer-term outcomes of hybrid ablative procedures are yet to be evaluated.
Conclusions
Our study offers the first insight into the benefits of hybrid thoracoscopic epicardial and catheter based endocardial ablation in obese patients with AF. The initial outcomes suggest that this novel technique may be efficacious in obese patients, however, the present results require validation with further studies of larger sample sizes with longer follow-up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: No ethical approval was required for the present study as the hybrid approach was standard of care in our institution and patients were not randomized. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Dietz WH. The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annu Rev Public Health 2015;36:575-96. [Crossref] [PubMed]
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25. [Crossref] [PubMed]
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471-7. [Crossref] [PubMed]
- Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol 2010;55:2319-27. [Crossref] [PubMed]
- Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J 2008;155:310-5. [Crossref] [PubMed]
- Wong CX, Sullivan T, Sun MT, et al. Obesity and the Risk of Incident, Post-Operative, and Post-Ablation Atrial Fibrillation: A Meta-Analysis of 626,603 Individuals in 51 Studies. JACC: Clinical Electrophysiology 2015;1:139-52.
- Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539-44. [Crossref] [PubMed]
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077-81. [Crossref] [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [Crossref] [PubMed]
- Pisters R, de Vos CB, Nieuwlaat R, et al. Use and underuse of oral anticoagulation for stroke prevention in atrial fibrillation: old and new paradigms. Semin Thromb Hemost 2009;35:554-9. [Crossref] [PubMed]
- Scherr D, Khairy P, Miyazaki S, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18-24. [Crossref] [PubMed]
- Phan K, Phan S, Thiagalingam A, et al. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardiothorac Surg 2016;49:1044-51. [Crossref] [PubMed]
- Phan K, Xie A, Kumar N, et al. Comparing energy sources for surgical ablation of atrial fibrillation: a Bayesian network meta-analysis of randomized, controlled trials. Eur J Cardiothorac Surg 2015;48:201-11. [Crossref] [PubMed]
- Phan K, Xie A, Tsai YC, et al. Biatrial ablation vs. left atrial concomitant surgical ablation for treatment of atrial fibrillation: a meta-analysis. Europace 2015;17:38-47. [Crossref] [PubMed]
- Phan K, Xie A, La Meir M, et al. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomised controlled trials. Heart 2014;100:722-30. [Crossref] [PubMed]
- Kearney K, Stephenson R, Phan K, et al. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann Cardiothorac Surg 2014;3:15-29. [PubMed]
- Phan K, Xie A, Tian DH, et al. Systematic review and meta-analysis of surgical ablation for atrial fibrillation during mitral valve surgery. Ann Cardiothorac Surg 2014;3:3-14. [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [Crossref] [PubMed]
- Stamou SC, Khabbaz KR, Mahmood F, et al. A multidisciplinary approach to the minimally invasive pulmonary vein isolation for treatment of atrial fibrillation. Ann Thorac Surg 2010;89:648-50. [Crossref] [PubMed]
- Xu J, Luc JG, Phan K. Atrial fibrillation: review of current treatment strategies. J Thorac Dis 2016;8:E886-E900. [Crossref] [PubMed]
- Kumar N, Bonizzi P, Pison L, et al. Impact of hybrid procedure on P wave duration for atrial fibrillation ablation. J Interv Card Electrophysiol 2015;42:91-9. [Crossref] [PubMed]
- La Meir M, Gelsomino S, Luca F, et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol 2013;167:1469-75. [Crossref] [PubMed]
- Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8. [Crossref] [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2014;45:401-7. [Crossref] [PubMed]
- Chilukuri K, Dalal D, Gadrey S, et al. A Prospective Study Evaluating the Role of Obesity and Obstructive Sleep Apnea for Outcomes After Catheter Ablation of Atrial Fibrillation. Journal of Cardiovascular Electrophysiology 2010;21:521-5. [Crossref] [PubMed]
- Jongnarangsin K, Chugh A, Good E, et al. Body Mass Index, Obstructive Sleep Apnea, and Outcomes of Catheter Ablation of Atrial Fibrillation. Journal of Cardiovascular Electrophysiology 2008;19:668-72. [Crossref] [PubMed]
- Mohanty S, Mohanty P, Di Biase L, et al. Impact of Metabolic Syndrome on Procedural Outcomes in Patients With Atrial Fibrillation Undergoing Catheter Ablation. Journal of the American College of Cardiology 2012;59:1295-301. [Crossref] [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid Thoracoscopic Surgical and Transvenous Catheter Ablation of Atrial Fibrillation. Journal of the American College of Cardiology 2012;60:54-61. [Crossref] [PubMed]
- Wong CX, Abed HS, Molaee P, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 2011;57:1745-51. [Crossref] [PubMed]
- Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90-100. [Crossref] [PubMed]
- Mahajan R, Lau DH, Brooks AG, et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. Journal of the American College of Cardiology 2015;66:1-11. [Crossref] [PubMed]
- Nalliah CJ, Sanders P, Kottkamp H, et al. The role of obesity in atrial fibrillation. European Heart Journal 2016;37:1565-72. [Crossref] [PubMed]
- Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol 2015;65:2159-69. [Crossref] [PubMed]
- Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc 2014;3:e000916. [Crossref] [PubMed]
- Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222-31. [Crossref] [PubMed]
- Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol 2007;20:89-93. [Crossref] [PubMed]
- Mehall JR, Kohut RM Jr, Schneeberger EW, et al. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg 2007;83:538-41. [Crossref] [PubMed]
- Beyer E, Lee R, Lam BK. Point: Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J Thorac Cardiovasc Surg 2009;137:521-6. [Crossref] [PubMed]
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7:835-46. [Crossref] [PubMed]
- Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460-6. [Crossref] [PubMed]
- Marcus GM, Smith LM, Ordovas K, et al. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm 2010;7:149-54. [Crossref] [PubMed]
- Mohanty S, Mohanty P, Di Biase L, et al. Influence of body mass index on quality of life in atrial fibrillation patients undergoing catheter ablation. Heart Rhythm 2011;8:1847-52. [Crossref] [PubMed]


