Surgical site infections after lung resection: a prospective study of risk factors in 1,091 consecutive patients
Introduction
The development of infectious complications after elective surgical procedures is a significant clinical burden. Postoperative infections are frequent and constitute 14–16% of all nosocomial infections (1). Prolongation of hospital stay and sometimes the need of intensive care unit treatment are relevant clinical and economic impacts of infectious complications. In thoracic surgery, the overall rate of postoperative infectious morbidity, including wound infection, pneumonia and empyema, has been reported in the range of 11% to 46% (2-5). Wide variation in these rates may partly be explained by the abundance of factors influencing infection development, and partly may be due to differences in definition criteria, completeness of recording and audit management of surgical site infection (SSI). The most widely used definitions for SSIs are those provided by the Centers for Diseases Control (CDC) in 1992, updated in 2003. Accordingly, surgical infections are commonly categorized into incisional and organ/space SSIs (6,7). In detail, the CDC defined incisional SSI those involving the skin and subcutaneous tissue (superficial incisional SSI) and those involving deep soft tissues of the incision (deep incisional SSI). Organ/space SSI involves any part of the organs or spaces, other than the incision, opened or manipulated during the operative procedure (6). The most frequent risk factors contributing to SSI can be grouped as patient-related or treatment-related (8). Well recognized patient-related risk factors are age and associated derangements of physiologic functions (especially respiratory function), low serum albumin level, diabetes mellitus and other metabolic disorders, advanced tumours and immunosuppressive diseases. Treatment-related factors that may increase the risk of SSI are steroids, radiation, chemotherapy, inadequate antibiotic prophylaxis, operative field contamination, duration of surgery, poor surgical technique. Awareness of these risk factors for SSI may help to identify patients undergoing lung resection who require special attention and additional perioperative measures to prevent complications.
The primary aim of this prospective study is to assess the incidence and the risk factors of SSI in a single-center series of patients undergoing lung resection over 10 years. The secondary aim is to compare the results of this study with the similar survey that had been carried out a decade earlier in our institution (9).
Methods
All patients undergoing lung resection at the Center for Thoracic Surgery of the University of Insubria in Varese, Italy, between January 2006 and December 2015, for non-infectious diseases and without signs of acute respiratory infections were eligible for this study. Patients receiving only explorative thoracotomy were excluded. During the 10-year period, we evaluated 1,091 consecutive patients (807 males, 284 females; mean age 59 years; median age 65 years) who underwent pulmonary resection. All patients were operated under general anaesthesia, with single-lung ventilation. The protocol of this study was in compliance with the local Institutional Review Board and received full approval. Surgical procedures were performed after obtaining written informed consent from all patients.
Antibiotic prophylaxis was ordered routinely for all patients, regardless of the type of lung resection. The antibiotic prophylaxis schedule was short-term and consisted of a single dose of ampicillin + sulbactam 3 g intravenously, 30 min before surgery. Generally, an additional antibiotic dose was given for prophylaxis if surgery lasted over 3 h. Patients did not receive the above prophylaxis, or received other types of antibiotic (fluoroquinolones), if antibiotic allergy was suspected or established.
Throughout the study surgeries were performed by members of our surgical team on rotation. All pneumonectomies were approached with open thoracotomy; lobectomies and wedged resections were performed with open thoracotomy or video-assisted thoracic surgery (VATS) access, according to the surgeon’s preference. The routine open approach for anatomical resection was lateral thoracotomy, through the fifth intercostal space, with minimal resection of latissimus dorsi, and anterior serratus preservation; at the end of the procedure two chest tubes were placed. Alternatively, we performed VATS lobectomy with three-port anterior approach (including a 4–6 cm mini-thoracotomy) and one chest tube anteriorly. Hilar-mediastinal lymphadenectomy was routinely performed. Wedge resections were managed either with three-port anterior approach or mini-thoracotomy according to surgeon’s preference.
In anatomical lung resection patients, standard postoperative analgesia was administered, including intravenous/oral paracetamol (1 g three times a day), epidural naropin (0.2% at 4 mL per h) and subcutaneous morphine (5 mg/day, at patient request); since postoperative day 4 until discharge: oral paracetamol/codeine (500/30 mg twice/day: at 6 am and 6 pm) and oral ibuprofen (600 mg twice/day: at 12 am and 12 pm). Supplemental analgesics [non-steroidal anti-inflammatory drugs (NSAID) and opioids] were available at patient request.
All patients underwent perioperative chest physiotherapy, including early postoperative care with standard measures of bronchial hygiene, lung rehabilitation by using respiratory exercisers (Coach®, Smiths Medical ASD Inc., Rockland MA, USA; PEP therapy), control of secretions and early ambulation. Moreover, to reduce risk of gastroesophageal reflux and aspiration we applied pre-emptive gastrointestinal tract management, as reported by Roberts (10), paying attention to fasting before and after surgery, used decontamination of the oropharynx and H2 antagonists or proton pump inhibitors.
For each patient the following data were prospectively collected: age, gender, blood haemoglobin concentration, blood total lymphocyte count, serum albumin concentration, forced expiratory volume in 1 second (FEV1) expressed as percentage of predicted value, history of diabetes, malignancy, induction chemo- and/or radiotherapy, preoperative steroid therapy, length of preoperative stay, antibiotic prophylaxis, type of surgical access (VATS/open thoracotomy), type of lung resection (pneumonectomy, lobectomy, wedge resection), operative time, length of postoperative stay. All these data and those of the recorded SSIs were stored by medical professionals in a dedicated computerized database (Microsoft Excel© , Microsoft Corp, Redmond, WA, USA), supervised by a data manager.
According to CDC definitions (6), we considered the following SSIs occurring within 30 days after lung resection: chest wound infection, ipsilateral pneumonia and ipsilateral empyema. Postoperative infections were prospectively recorded by daily inpatient visits until discharge, and subsequently by planned outpatient visits. Follow-up was complete for all 1,091 patients.
Wound infections were diagnosed according to clinical criteria, as described by Bailey et al. (11). Postoperative pneumonia was defined by clinical-radiological criteria: presence of new and/or progressive pulmonary infiltrates on chest X-rays, associated with fever (>38 °C), leukocytosis (white blood cell count >11,000/µL), purulent sputum, or isolation of pathogens in respiratory secretions. Postoperative empyema was diagnosed by radiological findings (pleural effusion with air-fluid levels) associated with symptoms and signs of infection (fever, leucocytosis) and/or isolation of pathogens in pleural fluid. All types of postoperative infections were initially treated with empiric antibiotic therapy, or according to susceptibility tests when available. In few cases, surgical treatment of infection was performed (wound debridement, abscess evacuation, thoracic drainage or re-VATS for pleural debridement/lavage) in addition to antibiotic therapy.
Statistical analysis
Results are expressed as mean value ± standard deviation (SD), or median value and interquartile range (IQR). Data were compared between groups using the χ2 test for categorical variables and Student’s t-test or Mann-Whitney U-test for continuous variables. In univariable and multivariable analysis the logistic regression model was used to evaluate the correlation between possible risk factors and SSI. For the multivariable logistic regression analysis, only variables with P≤0.15 at the univariable analysis and with sample size ≥872 patients (80% of the whole sample) were considered. A P value of <0.05 was considered significant. Statistical analysis of data was performed with MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium).
Results
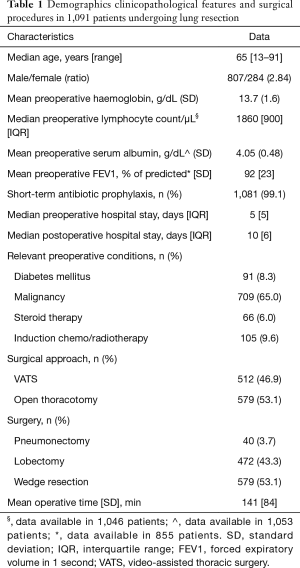
Demographics, clinicopathological features and surgical procedures for the 1,091 patients undergoing lung resection are summarized in Table 1. For all patients, the collection of information on risk factors was nearly complete. Missing data were: lymphocyte count in 45 patients (4%), serum albumin level in 38 patients (3.5%), FEV1 in 236 patients (21.6%, all undergoing lung wedge resection).
Full table
Of all procedures, 608 (56%) were performed for primary lung cancer, 100 (9%) for metastatic cancer and 383 (35%) for benign lung disease. Induction chemo and/or radiotherapy was administered in 105 patients undergoing respectively: wedge resection, n=30; lobectomy, n=56; pneumonectomy, n=19. VATS approach was used in 47% of patients, a lateral open thoracotomy in 53%. Distribution of surgical procedures was: 579 wedge resections (53.1%), 472 lobectomies (43.3%), 40 pneumonectomies (3.7%).
Short-term antibiotic prophylaxis was administered to 998 patients (91.5%) with the antibiotic of choice (ampicillin + sulbactam); in 83 patients (7.6%) we used other antibiotics because of allergy to penicillin (n=33), endocarditis prophylaxis (n=39), or other miscellaneous reasons (n=11); 10 patients (0.9%) received no antibiotic prophylaxis.
Table 2 summarizes the surgical infections observed during the 10-year survey. Overall, 124 patients (11.4%) developed one or more SSI; infection rates after wedge resection, lobectomy and pneumonectomy were respectively 4.8%, 17.4% and 35.0%.

Full table
Altogether we observed 147 SSIs. The incidence of wound infection, pneumonia and empyema by surgical procedure is detailed in Table 2. In order to evaluate the time trend of SSI over the last decade, patients were arbitrarily divided into two 5-year groups: group 1, operated in 2006–2010 (n=530); group 2, operated in 2011–2015 (n=561). These two groups were similar for clinicopathological characteristics and surgeries (data not shown). The SSI rate of group 1 (62/530, 11.7%) and that of group 2 (62/561, 11.1%) were not significantly different.
Postoperative death was recorded in 7 patients (0.6%) 4 of whom died of an infective postoperative complication (pneumonia in 3 cases; combination of pneumonia, empyema and wound infection in 1 case). The postoperative mortality rate was significantly higher after pneumonectomy (5.0%) compared to lobectomy (0.2%; P=0.006).
Patients who developed SSI, compared to those who did not, were significantly older (mean age: 65±13 vs. 58±18 years, P<0.001), more frequently male (85% vs. 73%, P=0.004), more frequently resected for malignancy (88% vs. 62%, P<0.001), had longer postoperative stay (17±10 vs. 10±6 days, P<0.001). Patients with SSI more frequently were operated by open thoracotomy than by VATS (76% vs. 50%, P<0.001), more often underwent major resection (lobectomy or pneumonectomy) relative to wedge (66%±11% vs. 40%±3%, P<0.001), and had longer mean operative time (194±85 vs. 134±81 min, P<0.001). Finally, postoperative mortality rate was higher in patients with SSI than in those without (4/124=3.2% vs. 3/967=0.3%, P<0.001). Between the two 5-year groups no significant differences were found in the incidence of wound infection, pneumonia and empyema (data not shown).
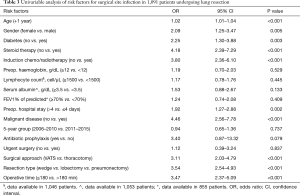
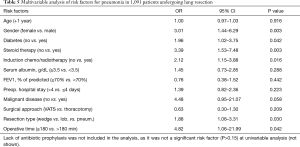
Results of univariable analysis of risk factors are reported in Table 3. Age, male gender, diabetes, steroid therapy, induction chemo/radiotherapy, preoperative hospital stay >4 days, malignant disease, open thoracotomy, type of surgical resection and operative time >180 min were risk factors for SSI. Multivariable analysis showed that male gender, diabetes, steroid therapy, induction chemo or radiotherapy, lack of antibiotic prophylaxis, and type of surgical resection were independent risk factors of overall SSI (Table 4). Six independent risk factors were associated with development of postoperative pneumonia in this series: male gender, diabetes, steroid therapy, induction chemo/radiotherapy, resection type and operative time (Table 5).
Full table

Full table
Full table
Discussion
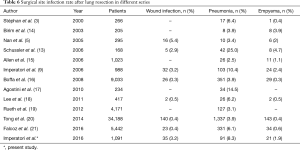
Mortality and morbidity after lung surgery have decreased over the last two decades because of improvements in surgical and anaesthesiology techniques, and better patient selection (12), nevertheless lung resection remains a risky procedure. Postoperative infections, cardiac events and respiratory failure are the main morbidities after thoracic surgery. The incidence of postoperative pneumonia, a dominating cause of death after pulmonary resection, has been reported as high as 25% (13) in the relevant series published in the years 2000–2016 (Table 6) (3,5,9,13-21). Lower rates of postoperative pneumonia were recorded in a survey by the Society of Thoracic Surgeons (3.9%) (16) and in a large cooperative multi-institutional investigation in the USA (2.5%) (15). The results of our study however indicate that infectious complications are frequent among patients undergoing lung surgery, as we found 11.4% incidence of overall SSIs and 8.3% of pneumonia. Compared with our previous survey (1996–2005) (9), the overall SSI incidence significantly decreased (14.3% vs. 11.4%, P=0.047), and there was a trend of pneumonia reduction from 10.4% to 8.3% (P=0.103); however, the incidences of wound infection and of empyema were broadly similar in the decades 1996–2005 and 2006–2015 (respectively 3.2% and 3.2%, P=0.964; 2.4% and 1.9%, P=0.430) (9). Interestingly, similar rates of wound infections after lung resection have been described in the literature in the last 2 decades (5,9,13,22,23).
Full table
The significant decrease of overall SSI incidence may be partly due to the significant increase use of VATS resections in the 2006–2015 decade (47% vs. 29%, P<0.001).
A methodological concern exists in comparing SSI results between the published reports, because of differences in data completeness, audit management, follow-up period, definitions and diagnostic criteria of infection. Thus, the results of single-center studies and those of cooperative multi-institutional or national databases may be contrasting. Moreover, a relevant proportion of postoperative infections develop after hospital discharge, and it has been emphasized that reliable methods for identifying post-discharge infections are necessary to capture all events (5). According to few thoracic surgeons, a controversial issue is grouping wound infection, pneumonia and empyema; however, including all these are in adherence to CDC definitions (6,7).
In the present series of 1,091 patients undergoing lung resections we confirm the negative impact of SSI on patient outcome, as mortality events were rare (0.6%), but frequently (57%) associated with postoperative infections.
Our results highlight six significant independent risk factors for SSI: male gender, diabetes, steroid therapy, induction chemo/radiotherapy, lack of antibiotic prophylaxis, major pulmonary resection. Some of these risk factors are sometimes modifiable (blood glucose control, antibiotic prophylaxis) and preventive measures should be implemented accordingly. Also, obesity (BMI ≥30 kg/m2), a potentially modifiable risk factor, in a prospective study was documented to be independently correlated with postoperative pulmonary complications (17); this relationship was not shown by others (24) and it was not investigated in the present study. It has been emphasized that obesity and FEV1 are risk factors subject to patient selection, and that results of analysis of their association with postoperative infections may be biased by selection (17).
The interpretation of the higher (twofold) SSI rate we observed in the male gender is puzzling. Consistent with our findings, other authors reported that male gender is associated with postoperative infection following resection of lung cancer (19) and other common solid tumours (25). Notably, a significantly higher rate of postoperative pneumonia was documented in the male gender in two large studies of pulmonary resections (13,26). Schussler et al. specified that the observed 25% incidence of postoperative pneumonia after major lung resections was independently associated with male gender, chronic obstructive pulmonary disease, greater extent of resection, and intraoperative bronchial colonization (13). In agreement with Simonsen et al. (26), our data show that a history of diabetes correlates with significantly higher risk of postoperative pneumonia. This correlation can partly be explained by the observation that hyperglycemia may negatively affect the innate immune system, especially neutrophil phagocytosis function, thus facilitating SSI (27). In addition, in long-standing diabetes a compromised microcirculation may play an important role favouring operative field infection.
Among treatment-related risk factors, the value of antibiotic prophylaxis remains an unsettled issue. Lung resection is classified as a clean-contaminated procedure and antibiotic prophylaxis in open thoracotomy is widely used. Controlled trials demonstrated significant reduction of wound infections in patients undergoing lung resection, but no effect on organ/space infections such as pneumonia or empyema (2). Our short-term antibiotic prophylaxis regimen (single dose of ampicillin + sulbactam intravenously), was in line with the American Society of Health System Pharmacists’ guidelines (28), and lack of antibiotic prophylaxis administration happened rarely. We found that lack of antibiotic prophylaxis was a factor independently associated with the occurrence of overall SSIs, while it was not a significant risk factor for empyema.
In our multivariable analysis, the FEV1 was not an independent predictor of SSI, a finding reported also by other authors who investigated the complications of lung resection (17,29). It must be noted that we did not have the FEV1 measurement available for 236 patients (all candidate to lung wedge resection); however, pneumonia developed only in 4 (1.7%) of these patients missing the FEV1 data, suggesting scarce correlation between infection and FEV1 with a cut-off of 70% of the predicted value. Extension and quality of surgical performance are relevant issues for SSI. Following the increased use of thoracoscopic anatomical lung resections worldwide in recent years, several studies compared the postoperative outcomes of open thoracotomy and VATS procedures. Jeon et al. reported a significantly lower incidence of pneumonia after VATS lobectomy compared to open lobectomy (30). It is generally agreed that lower morbidity after VATS lung resection is related to less trauma on the chest wall; however, multivariable analysis of our results does not indicate that the type of surgical access to the chest affects the incidence of overall SSIs. We hypothesize that in the causative mechanism of SSIs the duration and the quality of surgery play a greater role than the trauma of surgical access.
Based on risk stratification, models to predict complications can identify high-risk subjects (17). Postoperative complications however cannot be predicted in the individual patient, and predictive models should not be used to exclude candidates from surgery, as statistical association not necessarily indicates cause and effect relationship (17). Some prognostic variables may be modifiable and stratification by risk factors may serve as a guide to improve perioperative care protocols for lung resection (19).
Limitations and strengths
This study has limitations. First, this is a single-center study and the statistical analysis is retrospective, although the data were prospectively collected under regular revision by a data manager. Second, statistical analysis is limited by a large difference between the number of patients with and without SSI. Third, the association we observed between SSI and risk factors does not necessarily indicate causation, although other studies reported a similar correlation, because results may still be biased by unmeasured confounders. Fourth, we did not record all risk factors documented by other studies as BMI and transfer factor of the lung for carbon monoxide. Moreover, as already underlined, we did not have the FEV1 measurement available for approximately 22% of the patients. Finally, the cohort has had a long postoperative stay (median 10 days) that can be explained by our conservative attitude, poor district nurses support, and patient feeling to be safer in-hospital.
Points of strength of this study are the consecutive series and the prospective data collection in a dedicated database, all features that likely limited investigator bias. Other strengths are the adherence to CDC definitions, the large number of patients, all of whom had 30-day follow-up, and limited data missingness.
In conclusion, the observed 11.4% frequency of SSI in this study indicates that postoperative infections remain a relevant issue in lung resections. Focusing attention on identified and perioperatively modifiable risk factors of SSI may improve surgical results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The abstract of this article was presented at the 24th European Conference on General Thoracic Surgery 29 May–1 June 2016, Naples, Italy, European Society of Thoracic Surgeons.
Ethics statement: The protocol of this study was in compliance with the local Institutional Review Board and received full approval. Surgical procedures were performed after obtaining written informed consent from all patients.
References
- Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: National initiatives to improve outcomes for patients having surgery. Clin Infect Dis 2006;43:322-30. [Crossref] [PubMed]
- Bernard A, Pillet M, Goudet P, et al. Antibiotic prophylaxis in pulmonary surgery. A prospective randomized double-blind trial of flash cefuroxime versus forty-eight-hour cefuroxime. J Thorac Cardiovasc Surg 1994;107:896-900. [PubMed]
- Stéphan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- Turna A, Kutlu CA, Ozalp T, et al. Antibiotic prophylaxis in elective thoracic surgery: cefuroxime versus cefepime. Thorac Cardiovasc Surg 2003;51:84-8. [Crossref] [PubMed]
- Nan DN, Fernández-Ayala M, Fariñas-Alvarez C, et al. Nosocomial infection after lung surgery: incidence and risk factors. Chest 2005;128:2647-52. [Crossref] [PubMed]
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infection, 1992: A Modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606-8. [Crossref] [PubMed]
- Sands KE, Yokoe DS, Hooper DC, et al. Detection of postoperative surgical-site infections: comparison of health plan-based surveillance with hospital-based programs. Infect Control Hosp Epidemiol 2003;24:741-3. [Crossref] [PubMed]
- Dominioni L, Imperatori A, Rotolo N, et al. Risk factors for surgical infections. Surg Infect (Larchmt) 2006;7 Suppl 2:S9-12. [Crossref] [PubMed]
- Imperatori A, Rovera F, Rotolo N, et al. Prospective study of infection risk factors in 988 lung resections. Surg Infect (Larchmt) 2006;7 Suppl 2:S57-60. [Crossref] [PubMed]
- Roberts JR, Shyr Y, Christian KR, et al. Preemptive gastrointestinal tract management reduces aspiration and respiratory failure after thoracic operations. J Thorac Cardiovasc Surg 2000;119:449-52. [Crossref] [PubMed]
- Bailey IS, Karran SE, Toyn K, et al. Community surveillance of complications after hernia surgery. BMJ 1992;304:469-71. [Crossref] [PubMed]
- Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014;2:1007-15. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Lee JY, Jin SM, Lee CH, et al. Risk factors of postoperative pneumonia after lung cancer surgery. J Korean Med Sci 2011;26:979-84. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Tong BC, Kosinski AS, Burfeind WR Jr, et al. Sex differences in early outcomes after lung cancer resection: analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg 2014;148:13-8. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. ESTS Database Committee and ESTS Minimally Invasive Interest Group. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Rovera F, Imperatori A, Militello P, et al. Infections in 346 consecutive video-assisted thoracoscopic procedures. Surg Infect (Larchmt) 2003;4:45-51. [Crossref] [PubMed]
- Martin-Ucar AE, Aragon J, Bolufer Nadal S, et al. The influence of prior multiport experience on the learning curve for single-port thoracoscopic lobectomy: a multicentre comparative study. Eur J Cardiothorac Surg 2017;51:1183-7. [Crossref] [PubMed]
- Licker M, Spiliopoulos A, Frej JG, et al. Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest 2002;121:1890-7. [Crossref] [PubMed]
- Avritscher EB, Cooksley CD, Rolston KV, et al. Serious postoperative infections following resection of common solid tumors: outcomes, costs, and impact of hospital surgical volume. Support Care Cancer 2014;22:527-35. [Crossref] [PubMed]
- Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [Crossref] [PubMed]
- Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: Clinical, cellular, and molecular aspects. Crit Care Med 2005;33:1624-33. [Crossref] [PubMed]
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73-156. [Crossref] [PubMed]
- Brunelli A, Refai M, Monteverde M, et al. Stair climbing test predicts cardiopulmonary complications after lung resection. Chest 2002;121:1106-10. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]


