Contralateral thoracoscopic lobectomy in postlobectomy patients
Introduction
Computed tomography (CT) and high-resolution CT provide increasing opportunities to identify multifocal lung adenocarcinoma. Bilateral resection of synchronous or metachronous lung cancer had been well discussed in terms of surgery (such as the extent of resection, safety, and outcomes), and tumor origin (primary or metastasis) (1-3). However, little is known about the intraoperative management of bilateral lung resection.
Video-assisted thoracic surgery (VATS) for primary lung cancer was first reported in the early 1990s (4,5); since then, many reports have published the feasibility, safety, and less invasiveness of this procedure (6,7). For VATS procedures, stable oxygenation and sufficient collapse of the lung are very important. It is difficult to perform thoracoscopic lobectomy when perioperative stable oxygenation is not maintained in one-lung ventilation.
In this report, we investigated postlobectomy patients who could not maintain sufficient perioperative oxygenation following contralateral thoracoscopic lobectomy and discussed the predicted pulmonary function, including perioperative management of oxygenation.
Methods
Patients
Between 2008 and 2015, 14 patients underwent contralateral thoracoscopic lobectomy after lobectomy at the Department of Thoracic Surgery, Shin-kokura Hospital, Federation of National Public Service, Personnel Mutual Aid Associations, Japan. In accordance with internal guidelines, all of the patients were informed of the risks of dyspnea and associated complications after surgery and consented to their participation. The clinical data of the patients were retrospectively reviewed (Table 1).

Full table
Predicted pulmonary function before surgery
Before surgery, 14 patients who underwent contralateral thoracoscopic lobectomy after lobectomy were evaluated by ventilation/perfusion scintigraphy and pulmonary function tests. We calculated the predicted residual pulmonary function combined with technetium ventilation/perfusion scans from the ratio of right versus left fractional perfusion. The following formula was used for the predicted residual pulmonary function: Predicted residual pulmonary function = pulmonary function × (1 ‒ ratio of lung perfusion on the resected side × number of resected pulmonary segments/number of pulmonary segments on the resected side) (8). In our institution, lobectomy is indicated for patients with a predicted postoperative forced vital capacity (ppo FVC) of >800 mL/m2 or a predicted postoperative forced expiratory volume in one second (ppo FEV1) of >600 mL/m2 (8).
The six-minute walk test (6MWT) was performed in accordance with the guidelines of the American Thoracic Society (9,10).
Special oxygenation techniques
In cases who could not maintain stable oxygenation in contralateral residual one-lung ventilation, we performed the special oxygenation techniques during thoracoscopic lobectomy. A lobe-selective lung collapse technique was performed using an endobronchial blocker (Coopdech endobronchial blocker; Daiken Medical Co. Ltd., Osaka, Japan), as previously described (11). An endobronchial blocker tube was introduced into the lobar bronchus to be resected under bronchoscopy and performed via an endotracheal tube (Figure 1). The lobe to be resected collapsed after the balloon was inflated. The VATS procedure was started after confirmation of stable oxygenation (Figure S1).
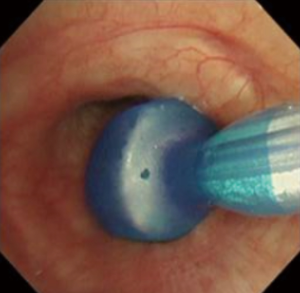

High-frequency jet ventilation (HFJV) using a MERA JP-1 device (Senko Medical Instrument Manufacturing, Saitama, Japan) was applied during the VATS procedure. A jet injector cannula with a 15-gauge was introduced into the operative side bronchus under bronchoscopy and performed via an endotracheal tube. HFJV was set at a frequency of 180 breaths per minute with a inspiratory-expiratory ratio of 0.5 and a driving pressure of 0.5–0.8 bar; the inspired oxygen concentration (FIO2) was set at 0.6 to 1.0.
Intermittent positive airway pressure to the operative side lung was performed under contra-residual lung ventilation. A 12-gauge suction tube was inserted into the operative side bronchus through an endotracheal tube and 2–5 L/min of oxygen was inflated into the operative side lung. When sufficient oxygenation could not be maintained, the VATS procedure was stopped, and operative side lung was reinflated to obtain stable oxygenation.
Whether or not the operation should be interrupted or continued was determined by the anesthesiologist at our hospital.
VATS procedure
The patient was placed in the lateral decubitus position. A 10 mm, 30-degree thoracoscope was placed through the seventh intercostal space in the midaxillary line. A 12 mm incision was made in the sixth or seventh intercostal space in the auscultatory triangle. Access thoracotomy was performed in the fourth intercostal space in the anterior axillary line (3 to 5 cm) (12).
Results
Patients who could not maintain stable oxygenation
We performed thoracoscopic lobectomy after contralateral lobectomy for 14 patients (Table 1). Among these patients, the preoperative pulmonary function, postoperative complication, and hospital stay differed between the special ventilation group and usual one-lung ventilation group. We performed thoracoscopic lobectomy after contralateral lobectomy for 14 patients (Table 2). Among 14 patients, 4 were unable to maintain sufficient perioperative oxygenation on contralateral usual one-lung ventilation. These four cases (cases No. 1–4) who underwent special oxygenation techniques had predicted postoperative FEV1.0 <800 mL/m2, 3 of 4 had mean preoperative 6MD ≤400 m, and ≤5 contralateral residual segments for ventilation. Cases No. 8 and 14 had predicted postoperative FEV1.0 <800 mL/m2 but >5 contralateral residual segments for ventilation. Although cases No. 9–13 also had ≤5 contralateral residual segments for ventilation, the predicted postoperative was FEV1.0 >800 mL/m2. These results suggested that both ppo FEV1.0 <800 mL/m2 and ≤5 contralateral residual segments for ventilation might be predictors for needing special oxygenation.
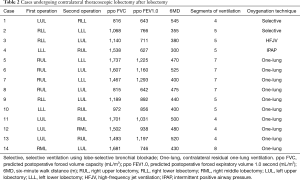
Full table
To obtain stable oxygenation and sufficient collapse of the lung for the VATS procedure, two patients underwent selective ventilation using lobe-selective bronchial blockade (cases No. 1,2) (Figure S1), one underwent HFJV (case No. 3), and one underwent IPAP for the operative-side lung (case No. 4). Because it is easier to obtain stable oxygenation and sufficient collapse of the lung in the VATS procedure than in HFJV, we attempted to perform a lobe-selective lung collapse technique for all cases. However, in cases No. 3 and 4, the endobronchial blocker tube could not be fixed into the planned resected lobar bronchus.
Discussion
Due to recent advances in imaging techniques, the rate of detection or resection of synchronous bilateral lung cancers has increased (1-3). VATS requires sufficient collapse of the affected side of the lung to obtain working space. However, some patients cannot maintain stable oxygenation during surgery under typical conditions of one-lung anesthesia. In particular, it is difficult to perform VATS lobectomy in patients who previously underwent contralateral lobectomy. The major finding of our present report was that the following two factors both affected the outcomes of contralateral thoracoscopic lobectomy for postlobectomy patients (I) predicted postoperative FEV1.0 <800 mL/m2; (II) ≤5 contralateral residual segments for ventilation. Despite the few cases available for evaluation, these are both important factors to consider when performing contralateral thoracoscopic lobectomy for postlobectomy patients.
The locations of the residual segments may be also important factors in patients who previously underwent contralateral lobectomy. As shown in Table 2, three out of four patients (cases No. 2–4) who underwent a special oxygenation techniques had received their first resections on the lower lobes, suggesting that ventilation/perfusion imbalance between the upper and lower lobes may also be important factors. However, few cases were available in the special oxygenation group, and further accumulation of clinical experience is needed.
The use of a lobe-selective lung collapse technique using an endobronchial blocker was previously reported in a VATS procedure (11). One of the advantages of bronchial blockers over a tracheal tube is the selective collapse of the entire lung (Figure S1), thereby making it simpler to obtain perioperative oxygenation. However, we were unable to affix the endobronchial blocker tube into the planned resected lobar bronchus in case Nos. 3 and 4. Blockade for the upper lobe may be difficult due to the angle and distance between the entrance to the lobe and subsequent branching. If patients have thoracoscopic upper lobectomy, then another strategy of ventilation should be prepared for subsequent contralateral postlobectomy. Another method for performing VATS procedures is HFJV (12), although only a few reports of its use in this manner have been published. One reason for this low rate of implementation is that anesthesiologists do not routinely perform HFJV through VATS procedures. Another reason is that PaCO2 elimination is predicted to be high through this operation. In the present report, although the patients were of advanced age, hypercapnia did not continue after HFJV. Our third option, IPAP, was easy to apply and required no special apparatus or technique. This method is very simple but may interfere with the VATS procedure when sufficient oxygenation cannot be maintained. Therefore, our preliminary strategy for thoracoscopic lobectomy in patients with a history of contralateral lobectomy is as follows: first, we must evaluate the lung function (ppo FEV1.0 and contralateral residual segments for ventilation) and location (plan for resection of the upper lobe or not). If upper lobe resection is considered, jet ventilation is prepared for special oxygenation. Second, anesthesia with one-lung ventilation using a blocker tube is started. If stable oxygenation is unsuccessful, special oxygenation is started for contralateral thoracoscopic lobectomy, as described.
In our 14 cases, thoracoscopic lobectomy was performed without significant postoperative complications (grade 2 or better under Clavien-Dindo Classification) and with no mortalities. In the special ventilation group, two patients had grade 1 complications. The patients in our report had a relatively long hospital stay, averaging 20.6 and 33.2 days (as shown in Table 1). A lengthy hospital stay was required for respiratory rehabilitation. However, all patients were discharged without home oxygen therapy. In the special ventilation group, two experienced no events after discharge (7 and 3 years), and two eventually died due to recurrence of lung cancer.
Several limitations associated with the present study warrant mention. First, the case numbers were too few for subgroup analyses. These data should therefore be interpreted carefully. Second, this is a retrospective report. These patients may represent a small population of postlobectomy patients whose clinical condition was good enough to permit surgery. Finally, this report was only performed to evaluate the lung function outcomes, and all of the patients who were initially considered for bilateral surgical resection were included. Further accumulation of prospective experience is required.
In conclusion, we reported two factors—the predicted postoperative FEV1.0, residual segments for ventilation—that were both important for affecting the outcome of contralateral thoracoscopic lobectomy for postlobectomy patients. To safely perform VATS procedures in such patients, preoperative discussion is needed regarding the method of oxygenation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no potential conflicts of interest to declare.
Ethical Statement: This work was approved by the Internal Review Board of the institution and written informed consent was obtained from all patients.
References
- Yasuda M, Nagashima A, Haro A, et al. How should synchronous multiple primary adenocarcinomas of the lung be resected? Ann Thorac Surg 2014;97:e151-3. [Crossref] [PubMed]
- Shah AA, Barfield ME, Kelsey CR, et al. Outcomes after surgical management of synchronous bilateral primary lung cancers. Ann Thorac Surg 2012;93:1055-60; discussion 1060. [Crossref] [PubMed]
- De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg 2008;34:1215-22. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52. [Crossref] [PubMed]
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Uramoto H, Nakanishi R, Fujino Y, et al. Prediction of pulmonary complications after a lobectomy in patients with non-small cell lung cancer. Thorax 2001;56:59-61. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- Irie M, Nakanishi R, Hamada K, et al. Perioperative short-term pulmonary rehabilitation for patients undergoing lung volume reduction surgery. COPD 2011;8:444-9. [Crossref] [PubMed]
- Nakanishi R, Shinohara S, Muranaka K, et al. Innovative techniques for thoracoscopic lobectomy in postpneumonectomy patients. J Thorac Cardiovasc Surg 2013;146:724-5. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Muranaka K, et al. Thoracoscopic carinal resection and reconstruction in a patient with mucoepidermoid carcinoma. J Thorac Cardiovasc Surg 2013;145:1134-5. [Crossref] [PubMed]
- Yasuda M, Nakanishi R, Shinohara S, et al. Performing thoracoscopic lobectomy for Case No.1. Asvide 2017;4:409. Available online: http://www.asvide.com/articles/1723


