Is chest tube drainage losing ground in management of patients with spontaneous pneumothorax?
Primary spontaneous pneumothorax (PSP) refers to a pneumothorax occurring with no precipitating factor in an otherwise normal lung parenchyma. It is usually a disease of the young age with it being more common in tall thin males. Secondary spontaneous pneumothorax (SSP) refers to a pneumothorax associated with an underlying lung disease and is usually a disease of the more elderly population with a significantly higher morbidity and mortality in comparison to primary pneumothorax.
The etiology of PSP remains uncertain. Classically described as owing to the rupture of bullae or “blebs”, the demonstration by Janssen et al. that those with a first episode of pneumothorax had no more anatomic abnormalities than those with recurrent pneumothoraces has lent doubt that bullae may be a major precipitating factor (1). Recent evidence with auto-fluorescent pleuroscopy does, however, suggest that some anatomic parietal pleural abnormality is a key-instigating factor (2). Associated medical conditions may include Marfan syndrome, Burg-Hogg-Dubé syndrome, thoracic endometriosis, and homocysteinuria.
SSPs are associated with an underlying medical condition. Although the recurrence rate is similar to PSP, mortality is higher due to the underlying pulmonary pathology and consequently decreased pulmonary reserve (3). Historically tuberculosis was the most common cause, however, more recently chronic obstructive pulmonary disease (COPD) is being cited as the most frequently associated lung disease with SSPs in 57% to 70% in some series (4,5).
The initial management of patients with pneumothorax includes observation (usually a small sized pneumothorax), needle aspiration NE and chest tube drainage CTD (which includes different size drains from pig-tails to large size drains). Occasionally, some centres choose to perform thoracoscopy (medical or surgical) as an initial management for spontaneous pneumothorax.
Although chest drain insertion is a common daily procedure performed across all hospitals for patients with spontaneous pneumothorax, this procedure is also associated with significant morbidity and occasional mortality. Incorrect insertion of a chest drain can have disastrous consequences. Perforation of both the right and left ventricle has been described. Examples of incorrect placement also included the pericardium with subsequent tamponade, intra-pulmonary including the contra-lateral hemi-thorax and the liver (6).
The evidence for needle aspiration NA as an initial treatment for spontaneous pneumothorax has been growing over the years. It is simple, safe and the learning curve for performing it is shorter than chest tube drainage. It can be performed in an out-patient setting and if patients are hospitalized, usually require a shorter hospital stay. Despite this, the guideline for using NA as an initial intervention is more evident in the European guidelines in comparison to the American guidelines for management of spontaneous pneumothorax.
The British Thoracic Society (BTS) guideline (3) and European Respiratory Society (ERS) task force statement (7) recommend aspiration as the first intervention, when needed, for all PSP without tension or hemodynamic instability. The BTS guideline is more modest for SSP: NA can be considered for symptomatic patients with small spontaneous pneumothorax in an attempt to avoid CTD. On the other hand, the American College of Chest Physicians (ACCP) guideline (8) does not suggest NA for any patients with spontaneous pneumothorax.
In a Cochrane review by Wakai et al. (9), there was no significant difference between simple aspiration and intercostal tube drainage for initial management of PSP regarding: immediate success rate, early failure rate, duration of hospitalization, 1-year success rate and number of patients requiring pleurodesis at 1 year. Simple aspiration was associated with a reduction in the per cent of patients hospitalized when compared with intercostal tube drainage. Again, another very recent meta-analysis by Kim et al. (10) comparing seven studies for initial management of PSP showed that recurrence rate of aspiration and intercostal tube drainage did not differ significantly while NA was associated with a shorter hospital stay. NA was however associated with inferior regarding early resolution of pneumothorax in comparison to CTD.
The apparent evidence that many centres (11,12) are deviating away from NA as an initial management for spontaneous pneumothorax despite the existing guidelines might reflect the historical link between the chest tube and the pathology of a pneumothorax or the inappropriate awareness of the current guidelines regarding initial management for spontaneous pneumothorax. Additionally, many physicians might feel the higher safety level of a chest tube drainage for patients with pneumothorax in achieving complete resolution and the inability to detect a continuing air leak in patients treated with NA. Failure to comply with the guidelines in choosing the initial method for management of pneumothorax can lead to more unnecessary complications associated with CTD.
In our experience, the rate of recurrence of a first attack of spontaneous pneumothorax treated by observation, NA or CTD is around 40–50% and usually occurs in the first year after the attack. This coincides with the classical rate of recurrence of 20–60% (13). Several studies (14,15) have chosen to treat patients with spontaneous pneumothorax with upfront thoracoscopic blebectomy/bullectomy and/or pleural abrasion/pleurectomy without inserting a chest drain. This practice is well established in patients with recurrent spontaneous pneumothorax or patients at considerable risk like patients with occupational hazards (divers, pilots etc.). Nevertheless, this practice is frequently used in patients with first attack pneumothorax to shorten hospital stay and to prevent the high recurrence rate associated with developing a second attack of pneumothorax. The high recurrence rate is unacceptable by many patients especially with high anxiety to insert another chest drain, patients who felt “they were about to die” with their first attack of pneumothorax or patients who live remotely from appropriate medical service.
The main limitation of routine thoracoscopy in patients with spontaneous pneumothorax is the scarcity of resources and expert surgeons who can provide the service instantly in comparison to chest tube insertion which is usually performed by junior staff from a variety of specialties and is usually available in an emergency department setting. Additionally, first line thoracoscopy should be offered only centres after long term follow up of the results in regards of rate of recurrence. Our 4-year rate of recurrence is only 2% for PSP which is encouraging to offer to patients in fear of recurrence without surgical intervention after their first attack.
Although apparently losing ground, chest tube drainage will always be indicated in a subset of patients to achieve a safe drainage for spontaneous pneumothorax. Patients who develop a tension spontaneous pneumothorax or suffer any hemodynamic stability during their attack of pneumothorax, patients with complete lung collapse due to pneumothorax, need for assisted ventilation, bilateral pneumothorax and patients who rupture big bullae and have a large air leak all are not candidates for needle aspiration. These are usually excluded in studies comparing NA and CTD as initial measures for managing spontaneous pneumothorax.
Thelle et al. (16) performed a randomized controlled study comparing needle aspiration NA versus chest tube drainage CTD in patients with spontaneous pneumothorax. A total of 128 patients were included in three Norwegian hospitals including 48 patients with SSP. The main outcome was duration of hospital stay while their secondary outcomes were immediate success, 1-week success rates and complication rate for each intervention.
Patients randomized to NA (65 patients) were treated by a maximum of two aspirations and adequate response was guided by a pneumothorax of less than 20% on an X-ray and absence of any symptoms of breathlessness. Absence of either criteria would shift the patient to having a CTD (31.2% of NA patients eventually needed CTD). Patients randomized to CTD (63 patients) had a 12–28 Ch chest tube connected to a chest drainage system inserted in the 4th or 5th intercostal space in the mid axillary line (the 5th space is always outside the safe triangle for CTD as recommended by the BTS guidelines).
They found a shorter hospital stay associated with NA 2.4 vs. 4.6 days (P<0.001). Immediate success rate was 69% for NA in comparison to 32% for CTD (P<0.001). There was no significant difference regarding 1-week success rate.
Regarding complications of intervention, there was no recorded complication in the NA while 24% of the CTD suffered from a minor major complication. One patient suffered from empyema which eventually leads to death. Sixteen (25%) needed insertion of another chest tube during their course of treatment. The rate of CTD complications and the need to insert another chest drain due to blockage/displacement is probably higher than other studies.
Patients with a recurrent episode of pneumothorax (33 patients) were also included in the study and this subgroup of patients also showed superior immediate success rates in favour of NA (64.7% vs. 18.8%; P=0.008) while no statistical significance could be recorded regarding hospital stay or 1-week success.
The authors concluded that starting intervention with NA in patients with both PSP and SSP (regardless of prior history of pneumothorax) lead to a shorter hospitalization stay and fewer complication than intervention with CTD and advised that the guidelines should be revised accordingly.
This is not the first study to show the efficacy and safety of NA in managing patients with spontaneous pneumothorax although this study is well designed and powered. Six randomized controlled studies (17-22) have preceded this. A summary of the results from the six studies is included in Table 1.
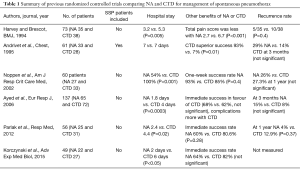
Full table
The evidence for NA for patients with SSP has been scarce in the literature but this study is the largest to examine patients with this more serious pathology. Only 1 of the previous 6 studies (18) looked at this and included only 8 patients with SSP. Thelle et al. are providing more confidence to the medical society to consider a simple NA for stable patients with SSP who had more benefit with NA regarding hospital stay (2.5 vs. 5.5 days; P=0.049) and immediate success (59.1% vs. 23.1%; P=0.011). This subgroup of patients is usually more morbid with less pulmonary/cardiac reserve and avoiding any potential complication from CTD would be advantageous.
A limitation in the study is the definition of immediate success for NA and CTD. For NA the authors have chosen to consider adequate response after two aspirations (success rate was high up to 68.8%) without considering failure of the first aspiration to be an inadequate response. CTD immediate success was based on removing the tube after 72 hours with resolution of the pneumothorax (resulted in success rate of only 31.8%; P<0.001). In terms of removing the chest drain, this is a brief period for patients with PSP in comparison to the BTS guidelines (5 days) and the ACCP guidelines (4 days). This might have resulted in the statistically significant difference found in all the subgroups of the study of a superior immediate success for NA in comparison to CTD while this is not evident in other studies while it is more logic that CTD will result in a higher chance of immediate resolution of a spontaneous pneumothorax in comparison to NA.
The deviation from time limit guidelines after using chest drain drainage in management for patients with spontaneous pneumothorax is evident in the medical society. We and others (11,12,23) have shown that this can be associated with a higher incidence of developing secondary complications to prolonged chest tube insertion; mainly in the form of developing an empyema which can further result in a longer hospital stay. I believe that avoiding using chest drains if possible in selected patients; as supported by this study; or adopting programs to perform a rapid thoracoscopy in patients with spontaneous pneumothorax can help to shorten hospital stay while a thoracoscopy is associated with the best chance to avoid the high chance of recurrence. Patients with recurrent stable pneumothorax in the study (33 patients) could have proceeded directly to thoracoscopy avoiding either interventions of NA or CTD.
To conclude, needle aspiration has gained reasonable evidence to replace chest tube drainage for initial treatment of stable patients with primary (and occasionally secondary) pneumothorax. It is safe and effective but carries a higher incidence of recurrence. Chest tube drainage can also be avoided in patients not accepting the high rate of recurrence of first attack spontaneous pneumothorax or in cases of recurrent pneumothorax and this group can be treated via upfront thoracoscopy in experienced centres. This management plan needs to be more clearly implemented in future published guidelines for management of spontaneous pneumothorax. Nevertheless, chest drains will always have a role in management of patients with spontaneous pneumothorax who are not candidates for needle aspiration or thoracoscopy and hence local and national training programmes should focus on allowing junior staff to insert chest drains safely.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Janssen JP, Schramel FM, Sutedja TG, et al. Videothoracoscopic appearance of first and recurrent pneumothorax. Chest 1995;108:330-4. [Crossref] [PubMed]
- Noppen M, Dekeukeleire T, Hanon S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med 2006;174:26-30. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J. BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Chen CH, Liao WC, Liu YH, et al. Secondary spontaneous pneumothorax: which associated conditions benefit from pigtail catheter treatment? Am J Emerg Med 2012;30:45-50. [Crossref] [PubMed]
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [Crossref] [PubMed]
- Harris A, O’Driscoll BR, Turkington PM. Survey of major complications of intercostal chest drain insertion in the UK. Postgrad Med J 2010;86:68-72. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Wakai A, O’Sullivan RG, McCabe G. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev 2007.CD004479. [PubMed]
- Kim MJ, Park I, Park JM, et al. Systematic review and meta-analysis of initial management of pneumothorax in adults: Intercostal tube drainage versus other invasive methods. PLoS One 2017;12:e0178802. [Crossref] [PubMed]
- Mendis D, El-Shanawany T, Mathur A, et al. Management of spontaneous pneumothorax: are British Thoracic Society guidelines being followed? Postgrad Med J 2002;78:80-4. [Crossref] [PubMed]
- Kelly AM, Clooney M. Spontaneous Pneumothorax Australia Study Group. Deviation from published guidelines in the management of primary spontaneous pneumothorax in Australia. Intern Med J 2008;38:64-7. [Crossref] [PubMed]
- Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax 1997;52:805-9. [Crossref] [PubMed]
- Al-Mourgi M, Alshehri F. Video-Assisted Thoracoscopic Surgery for the Treatment of First-Time Spontaneous Pneumothorax versus Conservative Treatment. Int J Health Sci (Qassim) 2015;9:428-32. [Crossref] [PubMed]
- Chiu CY, Chen TP, Wang CJ, et al. Factors associated with proceeding to surgical intervention and recurrence of primary spontaneous pneumothorax in adolescent patients. Eur J Pediatr 2014;173:1483-90. [Crossref] [PubMed]
- Thelle A, Gjerdevik M. Randomised comparison of needle aspiration and chest tube drainage in spontaneous pneumothorax. Eur Respir J 2017;49:1601296. [Crossref] [PubMed]
- Harvey J, Prescott RJ. Simple aspiration versus intercostal tube drainage for spontaneous pneumothorax in patients with normal lungs. British Thoracic Society Research Committee. BMJ 1994;309:1338-9. [Crossref] [PubMed]
- Andrivet P, Djedaini K, Teboul JL, et al. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest 1995;108:335-9. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J 2006;27:477-82. [Crossref] [PubMed]
- Korczyński P, Górska K, Nasiłowski J, et al. Comparison of Small Bore Catheter Aspiration and Chest Tube Drainage in the Management of Spontaneous Pneumothorax. Adv Exp Med Biol 2015;866:15-23. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Parlak M, Uil SM, van den Berg JW. A prospective, randomised trial of pneumothorax therapy: manual aspiration versus conventional chest tube drainage. Respir Med 2012;106:1600-5. [Crossref] [PubMed]
- Elsayed H, Kent W, McShane J, et al. Treatment of pneumothoraces at a tertiary centre: are we following the current guidelines? Interact Cardiovasc Thorac Surg 2011;12:430-3. [Crossref] [PubMed]


