Atypical carcinoid localized at the bronchus accompanied by diffuse idiopathic pulmonary neuroendocrine cell hyperplasia in the distal lung: a rare case report
Introduction
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is characterized by hyperplasia of noninvasive neuroendocrine cells. This is a rare pathological condition that, together with tumorlet, is suspected to be a precursor lesion of carcinoid (1). According to the WHO histological classification (4th edition) revised in 2015, DIPNECH was added to the category that includes neuroendocrine tumors (2). Here, a recent case of bronchial carcinoid occurring at the right intermediate trunk and accompanied by DIPNECH is reported.
Case presentation
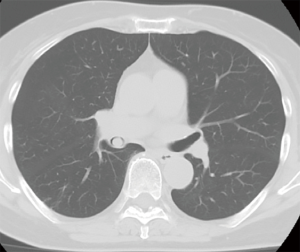
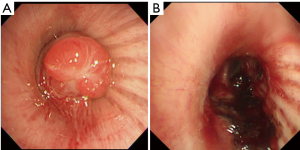
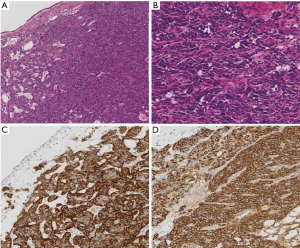
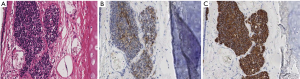
The patient was a 77-year-old woman who initially visited another doctor for the evaluation of a chronic dry cough. A chest CT examination revealed a polypoid mass occluding the right intermediate bronchial trunk, and a bronchoscopic examination was subsequently performed. Bronchial carcinoid was diagnosed based on the biopsy findings, and the patient was referred to our department for a more detailed examination. She had no history of smoking but had been treated for pulmonary tuberculosis. No major abnormalities were observed other than an elevated ProGRP level (234.6 pg/mL) upon hematological examination. The chest CT images showed a nodule with a diameter of 10 mm located at the bifurcation of the middle and lower lobe bronchi of the right intermediate trunk (Figure 1). The tumor occurring in the intermediate trunk was resected as completely as possible using a rigid bronchoscope to enable a definitive histological diagnosis and to determine the extent of tumor invasion. The tumor was observed from the bifurcation of the trachea. The tumor had a smooth surface and arose gradually from the membranous portion of the intermediate trunk located in the 6 o’clock direction. The airway appeared to be almost completely occluded when examined during the bronchoscopy, but aeration from the periphery was maintained. The tumor was excised en bloc using a high-frequency snare, and the stalk portion was cauterized using argon plasma coagulation (APC) and a diode laser (Figure 2). Histopathologically, hyperplasia of oval atypical cells with relatively poor cytoplasm was observed beneath the bronchial mucosa upon HE staining, suggesting a neuroendocrine tumor. Immunostaining revealed that these cells were positive for CD56, chromogranin A, and synaptophysin, and there were 4 mitoses per 2 mm2 (10 HPF), leading to a diagnosis of atypical carcinoid (Figure 3). Considering the histologic type of atypical carcinoid and the high ProGRP level, radical surgery comprised of a right pulmonary middle and lower lobectomy and lymph node dissection was performed based on the luminal findings obtained 2 months after the bronchial intervention. Histopathological examination of the excised lung specimen showed that atypical cells had formed multiple aggregations measuring approximately 1–3 mm along the airway around the bronchioles in the middle and lower lobes. On immunostaining, the atypical cells were positive for CD56, chromogranin A, and synaptophysin, but no mitoses were observed (Figure 4). Because the aggregations were multiple and distant from the tumor itself, metastasis was ruled out. Thus, a final diagnosis of atypical carcinoid (pT1aN0M0, stage IA) accompanied by DIPNECH was made. The patient had a favorable postoperative course, and was discharged 9 days after surgery. Among the tumor markers, ProGRP had decreased to 144.4 pg/mL at 3 months postoperatively, and remained at similar levels thereafter. The patient currently has no evidence of either recurrence or metastasis at 12 months after surgery, but meticulous follow-up examinations will be continued.
Discussion
Bronchial carcinoid is a relatively rare disease accounting for only 1–2% of all pulmonary malignant tumors, and is histologically classified into typical and atypical carcinoid according to the degree of cellular atypism, the rate of nuclear division, and the presence/absence of necrosis (3). In general, lymph node metastasis and distant metastasis of typical carcinoid are considered to be relatively rare. According to a report by Arrigoni et al. (4), the incidence of lymph node metastasis and distant metastasis was only 5.6%, and the 5-year survival rate was high, at 87%. On the other hand, lymph node metastasis or hematogenous metastasis occurs in 30–70% of patients with atypical carcinoid, and the 5-year survival rate is reportedly low, at 56%. Regardless of the histologic type, the first-line radical treatment is surgical resection. In the present case, double-barreled reconstruction of the right B6, B7-10, and intermediate bronchi was initially considered, but a right middle and lower lobectomy was eventually chosen, taking into account that the histologic type was atypical carcinoid and the ProGRP level was markedly elevated.
In this patient, DIPNECH was diagnosed based on the hyperplasia of neuroendocrine cells localized in the bronchioles, representing a pre-invasive lesion of a pulmonary malignant tumor. Since the first report by Aguayo et al. in 1992 (5), occasional case reports describing this condition have been reported. A relationship between DIPNECH and carcinoid has been suggested, and Miller et al. reported that concomitant DIPNECH was found in 76% of cases with peripheral-type carcinoid (6). Ruffini et al. identified DIPNECH in 0.28% of 1,090 patients who underwent surgery for lung cancer and in 5.7% of carcinoid cases (7). Davies et al. also reported that, among 19 DIPNECH cases, typical carcinoid was present in 47.4%, and atypical carcinoid was present in 15.8% (8).
DIPNECH was classified into the same major category (neuroendocrine tumors) as that of small cell carcinoma, large cell endocrine carcinoma, and typical and atypical carcinoid in the WHO histological classification (4th edition), revised in 2015 (2). DIPNECH is considered to be derived from Kultchitsky cells, and tumorlet and carcinoid are also considered to be lesions derived from these cells. Unlike tumorlets, which exhibit proliferation beyond the basal membrane, DIPNECH remains in the bronchial epithelium and does not invade the basal membrane. It is difficult to differentiate between tumorlets and carcinoids morphologically, and the WHO classification defines a tumorlet as having a diameter of less than 5 mm, while that of a carcinoid is 5 mm or more. These observations suggest that these lesions follow a developmental course from DIPNECH to tumorlet and finally to carcinoid, but whether this is the true continuum has yet to be determined. Further accumulation of cases is required to clarify this issue.
The presently reported patient was free of proliferation beyond the basal membrane and neither tumorlet nor carcinoid was present, based on the histopathological observations. However, the ProGRP level remained elevated, raising the possibility of minute DIPNECH in the remaining lung. Therefore, careful follow-up will be essential in this patient.
Conclusions
A case of atypical carcinoid accompanied by DIPNECH is reported. DIPNECH may be a precursor to carcinoid. Therefore, careful follow-up of this patient will be continued.
Acknowledgements
The authors are deeply grateful to Dr. Yasuhiro Terasaki (Department of Analytic Human Pathology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan), for both histopathologically reviewing this case and providing helpful advice.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Shotsu A, Maehara T, Adachi H, et al. A Case of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia with Peripheral Carcinoid Tumorlet. Haigan 2008;48:215-20. [Crossref]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Fraser RS, Mulleret NL, Colman NC, et al. Neuroendocrine neoplasms. In: Fraser RS. editor. Fraser and Pare’s Diagnosis of Diseases of the Chest. Philadelphia: WB Saunders Co, 1999;1229-50.
- Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg 1972;64:413-21. [PubMed]
- Aguayo SM, Miller YE, Waldron JA Jr, et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med 1992;327:1285-8. [Crossref] [PubMed]
- Miller RR, Müller NL. Neuroendocrine cell hyperplasia and obliterative bronchiolitis in patients with peripheral carcinoid tumors. Am J Surg Pathol 1995;19:653-8. [Crossref] [PubMed]
- Ruffini E, Bongiovanni M, Cavallo A, et al. The significance of associated pre-invasive lesions in patients resected for primary lung neoplasms. Eur J Cardiothorac Surg 2004;26:165-72. [Crossref] [PubMed]
- Davies SJ, Gosney JR, Hansell DM, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax 2007;62:248-52. [Crossref] [PubMed]


