Prognostic role of initial pan-endoscopic tumor length at diagnosis in operable esophageal squamous cell carcinoma undergoing esophagectomy with or without neoadjuvant concurrent chemoradiotherapy
Introduction
Esophageal squamous cell carcinoma (ESCC) is an aggressive malignancy with poor prognosis in Asian countries (1). The clinical-surgical-pathological T/N/M-status and cancer stage defined in the American Joint Committee on Cancer (AJCC) manual remained the cornerstone to predict survivals and tailor optimal treatment modalities for ESCC patients (2,3). Concerning the definition of T-status, the emphasis on tumor length (T1 or clinical stage I: tumor length ≤5 cm; T2 or clinical stage II: tumor length >5 cm length; T3 or clinical stage III: evidence of extra-esophageal spread; AJCC manual, 2nd edition, 1983) had been shifted to the depth of tumor invasion since 1988 (AJCC manual, 3–7th editions, 1988–2010) (4). Recently, the roles of tumor length in ESCC have been reappraised and some showed clinically relevance in the prediction of surgical resectability, survival outcomes, or acting as a criterion to select proper cases for neoadjuvant concurrent chemoradiotherapy (nCCRT) followed by surgical resection (5-13).
Multi-modal treatment modalities have been advocated to patients with esophageal cancer. Esophagectomy remained the optimal treatment modality for early-staged and resectable ESCC, and nCCRT followed by esophagectomy became a treatment of choice for advanced-staged but operable ESCC, depending on the clinical staging status (14). How to stage and distinguish early or advanced ESCC patients became an important task to both clinical oncologists and chest surgeons. According to the AJCC manual, several tools have been advocated to appraise the clinical stages of esophageal cancer as suggested by the Society of Thoracic Surgeons (15). Similar to other medical cancers in Taiwan, our institute, the Koo-Foundation Sun Yat-sen Cancer Center (KFSYSCC), mainly used pan-endoscopic examination with biopsy, computed tomography (CT) scan, and whole body bone can or fusion positron emission computed tomography (PET-CT) scan to access the clinical stages of ESCC (3,16-18).
Besides the clinical AJCC stages, initial pan-endoscopic tumor length at diagnosis was one of the selection criteria to figure out operable ESCC patients for upfront esophagectomy or nCCRT followed by esophagectomy in KFSYSCC during the past two decades (17,18). As a result, in this retrospective study, we would focus on the clinical relevance of initial pan-endoscopic tumor length at diagnosis in operable ESCC patients undergoing esophagectomy with or without nCCRT. Based on the initial pan-endoscopic tumor length at diagnosis (sub-groups ≤3, 3–5 or >5 cm), we wanted to evaluate the differences of survivals, clinical stages, pathological status and circumferential resection margin (CRM) status within ESCC patients undergoing upfront esophagectomy, within ESCC patients undergoing nCCRT followed by esophagectomy, or between ESCC patients undergoing upfront esophagectomy and nCCRT followed by esophagectomy, respectively.
Methods
Patient recruitment and primary treatment modality
Multi-modal treatment modalities, including surgical resection, chemotherapy, radiotherapy or their combinations, have been advocated for ESCC patients in KFSYSCC since 2000 (18). Routine work-up including pan-endoscopic examination with biopsy, CT scan from lower neck through thorax to abdomen, and whole body bone can or fusion PET-CT scan were arrange to evaluate the general oncologic condition. The clinical nodal (N) status was assessed mainly by either CT scan or PET-CT scan. Patients with initial pan-endoscopic tumor length at diagnosis ≤5 cm and without obvious lymph node involvement, i.e., the clinical nodal-negative N (−) status, would be treated with upfront surgical resection. Patients with initial pan-endoscopic tumor length at diagnosis >5 cm or with suspected lymph node involvement, i.e., the clinical nodal-positive N (+) status, would be treated with induction nCCRT followed by surgical resection if operable. Patients who had clinical stage IV or inoperable disease would undergo definite CCRT or palliative care. The criteria were minutely modified in 2009, wherein patients with initial pan-endoscopic tumor length at diagnosis ≤3 cm and without obvious clinical lymph node involvement would receive upfront surgical resection; whereas patients with initial pan-endoscopic tumor length at diagnosis >3 cm or with suspected clinical lymph node involvement would undergo nCCRT followed by surgical resection. As a result, when we discussed the initial pan-endoscopic tumor length of ESCC in this retrospective study, it was divided into 3 sub-groups including sub-group I ≤3 cm, sub-group II 3–5 cm and sub-group III >5 cm, respectively. A collaborative team would arrange the routine work-up and plan the suitable therapeutic strategy for ESCC patients. After discussing with ESCC patients, depending on their decisions and getting their written informed consents, specific primary treatment modality, including (I) surgery alone; (II) nCCRT followed by surgery; or (III) definite chemotherapy, radiotherapy or both, would be advocated for them. Post-operative adjuvant chemotherapy, radiotherapy or both would be given if the gross residual tumor or positive resection margin existed. Salvage chemotherapy, radiotherapy or both would be applied if recurrent or metastatic lesions developed during the follow-up periods (18).
From January 2001 to December 2013, a total of 229 ESCC patients without obvious clinical stage IV conditions and underwent esophagectomy were eligible for analysis in this retrospective study. Among them, 101 ESCC patients underwent upfront esophagectomy (surgery group) and 128 underwent nCCRT followed by esophagectomy (nCCRT-surgery group) as the primary therapeutic modality. Approval from the institutional review board of KFSYSCC was obtained to conduct this follow-up study (20150623A) and the informed consent requirement was waived.
Primary treatment modality
Surgery group (17)
The surgical approach contained transthoracic subtotal esophagectomy and lymph node dissection along the peri-esophageal region; gastric tube reconstruction after gastric cardiectomy and lymph node dissection along the left gastric artery to the main celiac trunk; and esophagogastric anastomosis through left cervical oblique incision and lymph node sampling if clinically suspected (17).
nCCRT-surgery group (18)
The nCCRT regimen has been reported previously (18). Concerning the chemotherapeutic agents, 5-fluorouracil (600 mg/m2/day) was administered for 2 divided 5 days from day 1 to 5 and from day 29 to 33, and cisplatin (60 mg/m2) was administered on day 1 and 29. Dosage may be escalated based on the patients’ tolerance and serum creatinine levels. Concerning the radiation therapy between day 1 and 33, it consisted of a total dose of 4,500 cGy, including 23 times of 180 cGy (divided daily fraction, 5 days per week, 23×180 cGy) to total field and 2 times of bolus 180 cGy to the tumor site (2×180 cGy). Esophagectomy would be performed 6–8 weeks after the completion of nCCRT (18).
Initial pan-endoscopic tumor length at diagnosis, clinical/pathological staging, pathological tumor length after esophagectomy
Based on the clinical-pathological findings, the clinical or pathological ESCC cancer stage was determined according to the T/N/M-status as described in AJCC manual, 7th edition (2). The pT/N/M-status or ypT/N/M-status were applied for surgery group or nCCRT-surgery group, respectively. Pan-endoscopic tumor length at diagnosis was recorded during the initial assessment. Pathological tumor length and the condition of the CRM invasion were evaluated in the pathological examination after esophagectomy.
Prognostic variables
Candidate variables including the initial pan-endoscopic tumor length at diagnosis (sub-group I ≤3 cm, sub-group II 3–5 cm and sub-group III >5 cm), CRM condition, and (y)pT/N/M-status and cancer stages were recorded in detail for comparison.
Statistical analysis
The overall survivals were calculated from the date of surgery in surgery group or date of induction nCCRT in nCCRT-surgery group (i.e., the date of the beginning of treatment) to the date of death or last follow-up till December 2013. Cox’s regression under continuous or categorical model (sub-group I ≤3 cm, sub-group II 3–5 cm and sub-group III >5 cm) was conducted to calculate the elevated hazard ratio (HR) regarding survival outcomes for the initial pan-endoscopic tumor length at diagnosis. Survival curve was plotted by the Kaplan–Meier method and the log-rank test was used to compare the survival differences among groups within the analyzed variable. Variables with a P value less than 0.10 were considered in a multivariate Cox’s regression analysis through the enter method. The categorical variables between two or among three or more groups were compared using the χ2 test/Fisher exact test or linear-by-linear association (χ2 test for trend) wherever appropriate. The continuous variables between two groups or among three or more groups were compared using t-test/Mann-Whitney U test or analysis of variance (ANOVA)/Kruskal-wallis H test when appropriate. Significance was assumed when a P less than 0.05.
Results
Demographic data of the ESCC patients in surgery group and nCCRT-surgery group
The demographic data of the 229 ESCC patients undergoing esophagectomy, including 101 in the surgery group (mean age of 57.5; 93 men) and 128 in the nCCRT-surgery group (mean age of 54.1; 121 men), are listed in Table 1. Concerning the distribution of initial clinical stages, there were 39 (38.6%) in stage I, 44 (43.6%) in stage II and 18 (17.8%) in stage III in surgery group, and 0 (0.0%) in stage I, 24 (18.8%) in stage II and 104 (81.3%) in stage III in nCCRT-surgery group, respectively. Significantly, surgery group had a higher percentage of early clinical stage I/II, on the contrary, nCCRT-surgery group had a higher percentage of advanced clinical stage III (P<0.001, footnoted) Their mean initial pan-endoscopic tumor length at diagnosis were 3.5 and 6.1 cm for surgery group and for nCCRT-surgery group, respectively; obviously, nCCRT-surgery group had a longer one (P<0.001, Table 1, footnotea). Besides, nCCRT-surgery group had higher percentage of sub-group III (>5 cm) ESCC patients than did surgery group (51.6% vs. 15.8%, P<0.001, Table 1, footnoteb). The mean pathological tumor length after esophagectomy were 3.5 and 1.6 cm for surgery group and for CCRT-surgery group, respectively; on the contrary, surgery group had a longer one (P<0.001, Table 1, footnotec). Eight (7.9%, 8/101) patients in surgery group and 12 (9.4%, 12/128) in the nCCRT-surgery group had CRM invasions. There were 54 (53.5%, 54/101) patients in surgery group and 95 (74.2%, 95/128) in nCCRT-surgery group diagnosed to be pN0 (ypN0) status without lymph node involvement. However, seven patients in the nCCRT-surgery group (5.5%, 7/128), including one belonged to sub-group II (3–5 cm) and six belonged to sub-group III (>5 cm), were proved to be ypT0N+ (positive nodal status with negative primary lesion, i.e., remnant lymph node metastasis following pathologic complete response of the primary tumor after nCCRT, Table 1, footnotee), which is not well documented in the current AJCC staging system (19,20). The mean/median survivals of the surgery group and of the nCCRT-surgery group were 80.7/72.5 and 80.1/70.2 months, respectively (Table 1).
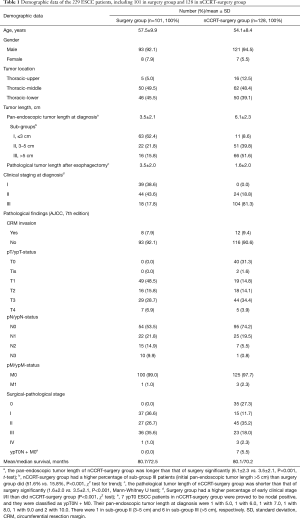
Full table
Prognostic role and HR of initial pan-endoscopic tumor length at diagnosis for ESCC patients
Longer initial pan-endoscopic tumor length at diagnosis was related to a higher HR of 1.209 in surgery group (continuous variable, Cox’s regression model, univariate, 95% CI, 1.091–1.339, P<0.001, Table 2, upper part) to cause poorer survival outcome. Among sub-groups I, II and III within surgery group, we found that sub-group III (>5 cm) had the highest HR of 4.967 (95% CI, 2.473–9.978, P<0.001) with the shortest survival of 38.3 months (95% CI, 10.8–65.8), followed by sub-group II (3–5 cm) of 1.912 (95% CI, 0.960–3.811, P=0.065) with intermediate survival of 61.3 months (95% CI, 38.7–83.9), and then sub-group I (≤3 cm) of 1.000 (as reference) with the longest survival of 96.2 months (95% CI, 78.5–113.8) in order (categorical variable, Cox’s regression model, univariate, P<0.001, for HR; log-rank test, P<0.001, for survival difference, Table 2, upper part). However, such a trend was not observed in nCCRT-surgery group (Table 2, lower part).

Full table
Prognostic factors in the surgery group
As shown in Table 3, longer initial pan-endoscopic tumor length at diagnosis (sub-groups ≤3, 3–5, and >5 cm; P<0.001), CRM invasion (P<0.001), advanced pT-status (P=0.007), advanced pN-status (P<0.001) and late cancer stage (P=0.002) were poor prognostic variables in surgery group. After Cox’s regression analysis through enter method, longer pan-endoscopic tumor length at diagnosis (sub-groups I, II, and III, ≤3, 3–5, and >5 cm; HR =1.000, 1.688, and 4.165; P=0.007) and CRM invasion (HR =5.152, P=0.028) were identified as independent variables to poor prognosis with elevated HRs within surgery group.
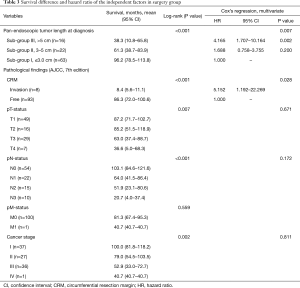
Full table
Prognostic factors in the nCCRT-surgery group
As listed in Table 4, CRM invasion (P=0.004), advanced ypT-status (P<0.001), advanced ypN-status (P=0.076), ypM1 status (P=0.011) and late cancer stage (P=0.003) were poor prognostic variables in nCCRT-surgery group. After Cox’s regression analysis through enter method, advanced ypT-status (T0, Tis, T1, T2, T3, and T4; HR =1.000, 1.954, 1.307, 0.938, 1.619, 21.584; P=0.025) was identified as an independent variable to poor prognosis with elevated HR in nCCRT-surgery group.
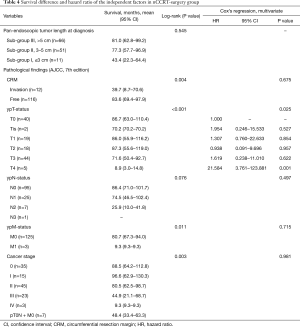
Full table
Impacts of initial pan-endoscopic tumor length at diagnosis (≤3, 3–5 and >5 cm) within or between surgery and nCCRT-surgery groups
Overall, there was no significant survival difference between the surgery group and the nCCRT-surgery group (80.7 vs. 80.1 months, Pa=0.934, Table 5). However, differences existed when they were divided in to sub-group I (≤3 cm), sub-group II (3–5 cm) and sub-group III (>5 cm) based on the initial pan-endoscopic tumor length at diagnosis (Table 5). Longer initial pan-endoscopic tumor length (≤3, 3–5, >5 cm) did associate with advanced clinical cancer stages (stages I, II, and III), regardless in surgery group (P<0.001) or in nCCRT-surgery group (P=0.035). Within surgery group or within nCCRT-surgery group, the survival differences among sub-groups I, II and III have been demonstrated in Tables 3,4, respectively. Concerning the sub-group I (≤3 cm), patients in surgery group had a better survival than did nCCRT-surgery group (96.2, 95% CI, 78.5–113.8 months vs. 43.4, 95% CI, 22.3–64.4 months, Pb=0.039). Concerning the sub-group III (>5 cm), patients in surgery group had a poorer survival than did nCCRT-surgery group (38.3, 95% CI, 10.8–65.8 months vs. 81.0, 95% CI, 62.8–99.2 months, Pd<0.001). Concerning the patients in sub-group II (3–5 cm), the survivals were not obviously different between surgery and nCCRT-surgery groups (61.3, 95% CI, 38.7–83.9 months vs. 77.3, 95% CI, 57.7–96.9 months, Pc=0.447).
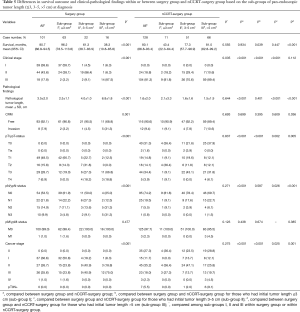
Full table
Concerning the condition of CRM invasion (Table 5), they were 7.9% (8/101) and 9.4% (12/128) in the surgery group and the nCCRT-surgery group, respectively; and no obvious difference was found (Pa=0.699). Within surgery group, from sub-group I (3.2%, 2/63) to sub-group II (4.5%, 1/22) and then sub-group III (31.3%, 5/16), a progressive increase in the rate of CRM invasion was noted (P*=0.001). However, such a trend among sub-groups I, II, and III was not found within nCCRT-surgery group (P*=0.693). For patients belonged to sub-group I (3.2%, 2/63 vs. 9.1%, 1/11, Pb=0.395) and sub-group II (4.5%, 1/22 vs. 7.8%, 4/51, Pc=0.609), the rate of CRM invasion between surgery and nCCRT-surgery groups were not obviously different. However, concerning the sub-group III (>5 cm) (31.3%, 5/16 vs. 10.6%, 7/66), the rate of CRM invasion in surgery group was higher than that of nCCRT-surgery group (Pd=0.036).
Within surgery group (Table 5, left part), from sub-group I, to sub-group II and further sub-group III, longer pan-endoscopic tumor length at diagnosis (≤3, 3–5, >5 cm) were related to advanced pT-status (P*<0.001), pN-status (P*<0.001) and cancer stage (P*<0.001). However, these trends about the progression of ypT-status (P*=0.837), ypN-status (P*=0.271) and cancer stage (P*=0.275) with their associations to initial pan-endoscopic tumor lengths were not observed within nCCRT-surgery group (Table 5, right part).
Concerning the distributive differences in (y)pT-status (Table 5), nCCRT-surgery group did have a higher percentage of pathological T0 status than did of surgery group (31.3%, 40/128 vs. 0.0%, 0/101; Pa<0.001), regardless the dividing into sub-group I (36.4%, 4/11 vs. 0.0%, 0/63; Pb<0.001), sub-group II (21.6%, 11/51 vs. 0.0%, 0/22; Pc=0.002) and sub-group III (37.9%, 25/66 vs. 0.0%, 0/16; Pd=0.005).
Concerning the distributive difference in (y)pN-status (Table 5), nCCRT-surgery group did have a higher percentage of pathological N0 status than did of surgery group (74.2%, 95/128 vs. 53.5%, 54/101; Pa<0.001). However, when dividing into sub-groups I, II and III, the differences were only observed in sub-group II (78.4%, 40/51 vs. 50.0%, 11/22; Pc=0.026) and in sub-group III (69.7%, 46/66 vs. 25.0%, 4/16; Pd<0.001), but not in sub-group I (81.8%, 9/11 vs. 61.9%, 39/63; Pb=0.587).
Concerning the distributive differences in cancer stage (Table 5), nCCRT-surgery group did have a higher percentage of pathological stage 0 than did of surgery group (27.3%, 35/128 vs. 0.0%, 0/101; Pa<0.001), regardless the dividing into sub-group I (36.4%, 4/11 vs. 0.0%, 0/63; Pb<0.001), sub-group II (23.5%, 12/51 vs. 0.0%, 0/22; Pc=0.025) and sub-group III (28.8%, 19/66 vs. 0.0%, 0/16; Pd=0.001). With regards to the 7 ypT0N+ ESCC patients (other than stage I–IV) in nCCRT-surgery group, 6 of the 7 had an initial pan-endoscopic tumor length exceeding 5 cm.
Discussion
Although a retrospective analysis, our results did offer new information about the initial pan-endoscopic tumor length at diagnosis in operable ESCC. In this study, we concluded that: (I) within surgery group, initial pan-endoscopic tumor length at diagnosis (≤3, 3–5, >5 cm) could not only predict the survival outcome but also be identified as an independent prognostic variable; (II) initial pan-endoscopic tumor length at diagnosis (≤3, 3–5, >5 cm) could be adopted as a criterion to select proper ESCC cases to receive nCCRT followed by esophagectomy to improve the survival outcome and pathological status, and to reduce the CRM invasion, especially for those who had an initial pan-endoscopic tumor length at diagnosis exceeding 5 cm (Table 5).
In concordance with several reported articles about tumor length in ESCC (11,12,21), we demonstrated that initial pan-endoscopic tumor length at diagnosis (≤3, 3–5, >5 cm) was an independent factor to predict survival outcome and to estimate relative risk within surgery group (Table 3). Besides, our results indicated that longer pan-endoscopic tumor length at diagnosis (sub-groups I, II and III, ≤3, 3–5 and >5 cm) was associated with CRM invasion (P=0.001), advanced pT-status (P<0.001), advanced pN-status (P<0.001) and late cancer stage (P<0.001) (Table 5, Left) within surgery group. It implied the that initial pan-endoscopic tumor length at diagnosis could be an efficient variable to reflect the, pathological CRM condition, pT-status, pN-status and cancer stage simultaneously, and could explain why it was identified as an independent variable within surgery group (Table 3) (10,12).
In 2000s, several original researches and review articles demonstrated survival benefit of nCCRT before esophagectomy for advanced ESCC (21,22). As a result, the advantages of nCCRT for advanced ESCC deserved to be discussed. In Table 1, as compared to surgery group (mean =3.5 cm; 15.8%, 16/101 belonged to sub-group III >5 cm), nCCRT-surgery group (mean =6.1 cm; 51.6%, 66/128 belonged to sub-group III >5 cm) had a significantly longer initial pan-endoscopic tumor length at diagnosis (P<0.001). It was assumed that nCCRT-surgery group would have a poorer survival and more advanced pT/N/M-status and cancer stage than did surgery group, if they all underwent upfront esophagectomy. An intriguing result showed that there was no significant survival difference between surgery (mean =80.7 months; 95% CI, 66.8–94.5) and nCCRT-surgery group (mean =80.1 months; 95% CI, 66.8–93.4; Pa=0.934) (Table 5). Furthermore, nCCRT-surgery group had shorter pathological tumor lengths (1.6 vs. 3.5 cm, Pa<0.001), higher percentage of T0 status (Pa<0.001), N0 status (Pa<0.001) and cancer stage 0 (Pa<0.001) than did surgery group (Table 5), as compared to surgery group. The distribution of survival and ypT/N/M-status and cancer stage in nCCRT-surgery group were better than expected. In this retrospective study, we did not perform propensity score matching between surgery group and nCCRT-surgery group during comparative analysis. However, the results did offer us important information. Long-term follow-up and increasing accumulated sample sizes might be necessary in the future. Nevertheless, the pathological patterns of esophageal cancer between the Western and Asian, including China, Korea, Japan and Taiwan, are quite different. Adenocarcinoma of the lower esophagus to the gastroesophageal junction (including the proximal gastric cardiac portion) is prevalent in the Western, and squamous cell carcinoma of the thoracic esophagus is relatively prevalent in Asian (23). As a result, the regimen of the nCCRT conducted for esophageal cancer in the Western, e.g., CROSS or MAGIC trials (24,25), are somewhat different as conducted in Asian, including our institute (18,26). However, they all emphasize the role of nCCRT to improve survival outcomes after esophagectomy for advanced esophageal cancer.
In the 2nd vision of AJCC manual, tumor length exceeding 5 cm indicated advanced clinical stage and low rate of surgical resectability (4). Concerning the clinical practice and therapeutic guideline set in our cancer center (KFSYSCC) in 2001, patients with pan-endoscopic tumor length exceeding 5 cm (which was mutely changed to >3 cm in 2009) were highly recommended to receive induction nCCRT before surgical resection rather than upfront surgical intervention (18). As a result, we were interested in the differences about the distribution of clinicopathological variables between surgery and nCCRT-surgery groups when they were divided into sub-groups I, II and III based on initial pan-endoscopic tumor length at diagnosis of ≤3, 3–5, >5 cm, respectively. Concerning the survival outcomes between the surgery group and nCCRT-surgery group, a reverse phenomenon was observed; for sub-group I (≤3 cm), surgery group had a better survival than did nCCRT-surgery group (Pb=0.039); for sub-group II (3–5 cm), surgery group and nCCRT-surgery group had similar survivals (Pc=0.447); for sub-group III (>5 cm), nCCRT-surgery group had a better survival than did surgery group (Pd<0.001). Except the distribution of N-status in sub-group I (≤3 cm, Pb=0.587), nCCRT-surgery group had higher rates of T0 status, N0 status and cancer stage 0 than did surgery group, no matter in sub-groups I, II and III (≤3, 3–5, >5 cm; all Pb, Pc, Pd<0.05) (Table 5). These novel findings made us conclude that ESCC patients with initial pan-endoscopic tumor length at diagnosis exceeding 5 cm did be benefit from nCCRT followed by surgical resection rather than upfront surgery. This result was also compatible with a population-based study in Taiwan (27). Initial pan-endoscopic tumor length at diagnosis of 5 cm may be an optimal criterion to select proper candidates for nCCRT followed by surgery or upfront surgical intervention.
However, there were some exceptions happened. As shown in Table 5, 11 of the nCCRT-group were belonged to sub-group I (≤3 cm), and they were supposed to undergo upfront esophagectomy, on the contrary, 16 of the surgery group were belonged to sub-group III (>5 cm), and they were supposed to undergo nCCRT followed by esophagectomy. As a result, we were interested in whether or not we followed the guideline to a certain extent in our institute. Through receiver operating characteristic curve (ROC curve) for our cohort of total 229 ESCC patients, initial pan-endoscopic tumor length at diagnosis of 5 cm (sensitivity of 0.83; specificity of 0.78; largest Youden Index of 0.61) is the best optimal cutoff value to distinguish whether the ESCC patients underwent upfront esophagectomy or nCCRT followed by esophagectomy. This cutoff value of 5 cm is compatible with the guideline we setup. In other words, our team did follow the therapeutic guideline without obvious deviation.
The reasons why nCCRT-surgery group had a worse outcome than did the surgery group among sub-group I (≤3 cm) ESCC patients deserved discussed. The findings were compatible with other’s reports that nCCRT is not beneficial and may be harmful in patients with earlier stage esophageal cancer (28,29). Another reason might be more cases harbored higher initial clinical stages in sub-group I of nCCRT-surgery group. Considering sub-group I (≤3 cm) ESCC patients, only 2 (3.2%) of the 63 in surgery group were in clinical stage III, however, 9 (81.8%) of the 11 in nCCRT-surgery group were in clinical stage III (Pb<0.001, Table 5). The above reasons might account for the clinical results. However, more sample populations were needed to validate in the future.
Consistent with the reported literature, CRM invasion did contribute to shorter survival in ESCC patients, either in surgery group or in nCCRT-surgery group (Tables 3,4) (18,30,31). When divided into sub-groups I, II and III (≤3, 3–5, >5 cm), the CRM invasion rates in surgery group raised from 3.2% to 4.5% and then 31.3% progressively, however, they remained around 10% in nCCRT-surgery group (Table 5).Significantly, among sub-group III ESCC patients, nCCRT-surgery group had a lower rate of CRM invasion than that of surgery group (10.6% vs. 31.3%, Pd=0.036). As a result, we concluded that initial pan-endoscopic tumor length at diagnosis may help us to select operable ESCC candidates with advanced stages for nCCRT to reduce the rate of CRM invasion during esophagectomy.
In surgery group, the pathological CRM condition, pT/N/M-status, cancer stage and pathological tumor length were all obtained after surgical intervention, and their severity could be reflected by initial pan-endoscopic tumor at diagnosis effectively (Table 5, left part). The role of initial pan-endoscopic tumor at diagnosis should be emphasized. By means of initial pan-endoscopic tumor length at diagnosis, we could figure out some operable ESCC patients to undergo nCCRT followed by esophagectomy, especially those harboring tumor length exceeding 5 cm that may be diagnosed as advanced pT/N/M-status and cancer stage if receiving upfront surgical resection. nCCRT may make the shrinkage of the tumor length to achieve lower rate of CRM invasion, down-staged ESCC lesions and better survivals.
ypT0N+ is a rare condition after nCCRT and only few articles describe about it (19,20). Why the residual lymph nodes involvement existed after nCCRT remained a puzzle. And we were curious of its relationship to initial pan-endoscopic tumor length at diagnosis. Among the 40 ypT0 ESCC patients in nCCRT-surgery group (Table 5), 33 achieved a ypN (−) status and 7 remained a ypN (+) status after nCCRT. Concerning the distribution of initial pan-endoscopic tumor lengths of the 7 pT0N+ patients, there were 6 in sub-group III (>5 cm) and 1 in sub-group II (3–5 cm) (Table 5). Furthermore, the mean initial pan-endoscopic tumor length of the 7 ypT0N+ patients was significantly longer than that of the 33 pT0N0 patients (7.9±2.0 vs. 5.9±1.9, P=0.034 Mann-Whitney U test, data not shown). The result indicated that longer initial pan-endoscopic tumor length may be a factor related to pT0N+ status after nCCRT. An increase of the radiation or chemotherapy dosage might be a therapeutic concern, however, we need more clinical data to validate this hypothesis in the future.
Advanced T status (T3/T4) or N (+) are important determinants to select proper ESCC patients for nCCRT followed by esophagectomy. Endoscopic ultrasound (EUS) is an important recommended staging algorithm in guidelines published by National Comprehensive Cancer Network (NCCN) (32-34). Nevertheless, in the current study, we mainly relied the initial pan-endoscopic tumor length or pre-operative PET/CT or CT scan to select advanced staged ESCC patients (16,18,35). Compared to EUS, several issues deserved to be discussed. Concerning the initial pan-endoscopic tumor lengths at diagnosis in surgery group to predict T-status, for those harboring ≤3 cm (sub-group I, n=63), >3 cm (sub-groups II and III, n=22+16=38) or >5 cm (sub-group III, n=16), there were 51 (51/63, 81.0%) proved to be T1/T2 status, 24 (24/38, 63.2%) proved to be T3/T4 status and 14 (14/16, 87.5%) proved to be T3/T4 status, respectively. With regards to sub-group I (≤3 cm) or sub-group III (>5 cm) in our cohort, their rates to identify early T1/T2 lesions or advanced T3/T4 lesions were 81.0% and 87.5%, respectively, and they were similar to the rates of EUS as reported, ranging from 81.0–90.0% (36,37). However, the role of >3 cm (sub-groups II and III) to identify advanced T3/T4 lesions was only 63.2%, thus, the aid of EUS seemed mandatory. Concerning the role of initial pan-endoscopic tumor lengths at diagnosis in surgery group to predict N-status, for those harboring ≤3 cm (sub-group I, n=63) or >3 cm (sub-groups II and III, n=22+16=38), there were 39 (39/63, 61.9%) proved to be N0 and 23 (23/38, 60.5%) proved to be N+. However, their rates to identify N0 or N (+) status were lower than that of EUS as reported of 74.0%, ranging from 62.0-83.0% or more higher (38,39). Interestingly, for those harboring initial pan-endoscopic tumor lengths at diagnosis >5 cm (sub-group III, n=16), there was 12 (12/16, 75%) proved to be N+, and it seemed similar to EUS. However, if fine needle aspiration (FNA) was applied simultaneously with EUS, the accurate rate to detect N-status may reach 83.0–97.0% (38-40). Taken together, EUS screening seemed necessary for ESCC patients. As a result, we started to use EUS to evaluated clinical T and N status in the recent 2 years in our institute since 2015. An appraisal of the results of PET/CT/EUS in ESCC patients who underwent upfront esophagectomy or nCCRT followed by esophagectomy in our cancer center might be an important issue in the future.
In conclusion, the initial pan-endoscopic tumor at diagnosis played dual roles in operable ESCC patients. It could predict the prognosis of ESCC patients who underwent primary surgical intervention. Besides, it could be a criterion to select proper operable ESCC patients, especially those harboring initial pan-endoscopic tumor at diagnosis exceeding 5 cm, for nCCRT followed by surgery to improve the survivals and pathological status, and to reduce the rate of CRM invasion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval from the institutional review board of KFSYSCC was obtained to conduct this follow-up study (20150623A) and the informed consent requirement was waived.
References
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer 2012;31:281-6. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Lin CS, Chang SC, Wei YH, et al. Prognostic variables in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2009;87:1056-65. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin 2013;23:461-9. [Crossref] [PubMed]
- Griffiths EA, Brummell Z, Gorthi G, et al. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol 2006;93:258-67. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Bolton WD, Hofstetter WL, Francis AM, et al. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2009;138:831-6. [Crossref] [PubMed]
- Bollschweiler E, Baldus SE, Schröder W, et al. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol 2006;94:355-63. [Crossref] [PubMed]
- Yendamuri S, Swisher SG, Correa AM, et al. Esophageal tumor length is independently associated with long-term survival. Cancer 2009;115:508-16. [Crossref] [PubMed]
- Gaur P, Sepesi B, Hofstetter WL, et al. Endoscopic esophageal tumor length: a prognostic factor for patients with esophageal cancer. Cancer 2011;117:63-9. [Crossref] [PubMed]
- Wang BY, Goan YG, Hsu PK, et al. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg 2011;91:887-93. [Crossref] [PubMed]
- Wang BY, Liu CY, Lin CH, et al. Endoscopic tumor length is an independent prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol 2012;19:2149-58. [Crossref] [PubMed]
- Barbour AP, Jones M, Gonen M, et al. Refining esophageal cancer staging after neoadjuvant therapy: importance of treatment response. Ann Surg Oncol 2008;15:2894-902. [Crossref] [PubMed]
- Wiedmann MW, Mössner J. New and emerging combination therapies for esophageal cancer. Cancer Manag Res 2013;5:133-46. [Crossref] [PubMed]
- Varghese TK Jr, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg 2013;96:346-56. [Crossref] [PubMed]
- Hsu WH, Hsu PK, Wang SJ, et al. Positron emission tomography-computed tomography in predicting locoregional invasion in esophageal squamous cell carcinoma. Ann Thorac Surg 2009;87:1564-8. [Crossref] [PubMed]
- Lin CS, Cheng CT, Liu CY, et al. Radical Lymph Node Dissection in Primary Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg 2015;100:278-86. [Crossref] [PubMed]
- Liu CY, Wang BY, Lee MY, et al. The prognostic value of circumferential resection margin in esophageal squamous cell carcinoma after concurrent chemoradiation therapy and surgery. J Chin Med Assoc 2013;76:570-5. [Crossref] [PubMed]
- Cho HJ, Kim YH, Kim HR, et al. Oncologic Outcomes According to Remnant Lymph Node Metastases in Pathologic T0 (ypT0) Esophageal Squamous Cell Carcinoma Following Prospective Neoadjuvant Therapy and Surgery. Ann Surg Oncol 2015;22:1851-7. [Crossref] [PubMed]
- Chao YK, Chen HS, Wang BY, et al. Prognosis of Patients With Pathologic T0 N+ Esophageal Squamous Cell Carcinoma After Chemoradiotherapy and Surgical Resection: Results From a Nationwide Study. Ann Thorac Surg 2016;101:1897-902. [Crossref] [PubMed]
- Murakami M, Kuroda Y, Okamoto Y, et al. Neoadjuvant concurrent chemoradiotherapy followed by definitive high-dose radiotherapy or surgery for operable thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 1998;40:1049-59. [Crossref] [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [Crossref] [PubMed]
- Brown LM. The role of race/ethnicity in the epidemiology of esophageal cancer. J Assoc Acad Minor Phys 2000;11:32-7. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Li CY, Huang PM, Chu PY, et al. Predictors of Survival in Esophageal Squamous Cell Carcinoma with Pathologic Major Response after Neoadjuvant Chemoradiation Therapy and Surgery: The Impact of Chemotherapy Protocols. Biomed Res Int 2016;2016:6423297. [Crossref] [PubMed]
- Chen HS, Wu SC, Hsu PK, et al. The Prognostic Impact of Preoperative and Postoperative Chemoradiation in Clinical Stage II and III Esophageal Squamous Cell Carcinomas: A Population Based Study in Taiwan. Medicine (Baltimore) 2015;94:e1002. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Effectiveness of neoadjuvant chemoradiotherapy for early-stage esophageal cancer. J Clin Oncol 2015;33:288-9. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Chao YK, Yeh CJ, Chang HK, et al. Impact of circumferential resection margin distance on locoregional recurrence and survival after chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol 2011;18:529-34. [Crossref] [PubMed]
- Pultrum BB, Honing J, Smit JK, et al. A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann Surg Oncol 2010;17:812-20. [Crossref] [PubMed]
- Ajani JA, D’Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw 2011;9:830-87. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6 Suppl 3:S289-97. [PubMed]
- Fujiwara Y, Yoshikawa R, Kamikonya N, et al. Neoadjuvant chemoradiotherapy followed by esophagectomy vs. surgery alone in the treatment of resectable esophageal squamous cell carcinoma. Mol Clin Oncol 2013;1:773-9. [Crossref] [PubMed]
- Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535-9. [Crossref] [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. [Crossref] [PubMed]
- Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology 2003;125:1626-35. [Crossref] [PubMed]
- Murata Y, Ohta M, Hayashi K, et al. Preoperative evaluation of lymph node metastasis in esophageal cancer. Ann Thorac Cardiovasc Surg 2003;9:88-92. [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]


