Lung cancer surgery: an up to date
Introduction
According to the International Agency for Research on Cancer (IARC) GLOBOCAN World Cancer Report (1), lung cancer affects more than 1 million people a year worldwide, holding, by far, the first place among other forms of cancer incidence and mortality in the global male population in women, it is the fourth most frequently occurring malignant tumour-after breast cancer, cervical and colorectal cancer-but it’s the first in mortality rates (1).
In Greece according to the 2008 GLOBOCAN report, there were 6,667 recorded cases, 18% of the total incidence of all cancers in the population. Furthermore, there were 6,402 deaths due to lung cancer, 23.5% of all deaths due to cancer. In men the rates are important. Lung cancer occupies by far the leading position both in incidence rates—5,540 new cases, 26.3% of the total of all cancers in Greece—and in mortality rates—5,321 deaths, a figure that exceeds 32% of all cancer deaths in 2008. Therefore lung cancer is the most common and deadly form of cancer in our country for the male population.
In women, incidence rates are alarming. Lung cancer ranks third after breast cancer and colorectal cancer, but found extremely high mortality. Specifically, there were 1,127 new cases of lung cancer, 7% of all cases of cancer among Greek women in 2008 and 1,081 deaths, a figure that exceeds 10.2% of all deaths due to cancer in that same year.
The treatment for lung cancer depends upon tumour histology (small cell versus non-small cell), extent (stage) and patient specific factors (e.g., age, pulmonary function, comorbidity). The major subtypes of non-small cell lung cancer (NSCLC) include adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, in decreasing order of frequency of occurrence.
In recent years there has been great progress in surgical technique and in the art of anaesthesia for lung surgery. Surgical therapy remains the cornerstone in the treatment of lung cancer and is the only way to address that offers long-term survival, at least in terms of patients with early-stage cancer and others who are at more advanced stages, after a very rigorous selection.
The surgical treatment of patients with stage I and II NSCLC
Staging of malignant tumours was a European initiative. Pierre Denoit [1912-1990], a surgical oncologist at Gustave-Roussy Institute in Paris, first started using the tumour, node, metastasis (TNM) classification system to describe the anatomic extent of disease (2). Later on, the International Union against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) adopted this system. For lung cancer, however, the big push came from the United States of America with Clifton Mountain, whose database served several editions and revisions of the TNM classification till the 6th edition (3).
In 1996, another European, Mr. Peter Goldstraw, a thoracic surgeon from the Royal Brompton Hospital, London, UK, lead the initiative to collect a large, international database, within the International Association for the Study of Lung Cancer (IASLC). He was the first Chairman of the IASLC International Staging Committee, and lead all the work of the committee resulting in several data-based recommendations to modify the 6th edition of the TNM classification, that were fully accepted by the UICC and the AJCC. The result was the revised 7th edition, the text of which is identical to the staging manuals of the IASLC, the UICC and the AJCC (4,5). Nearly 60% of the more than 100,000 cases registered in the IASLC database, were from European institutions, which gives evidence of the interest of the continent in lung cancer staging (6).
Surgery is the standard treatment for patients with clinical stage I and II NSCLC (Tables 1,2) in whom there is no evidence of mediastinal involvement prior to surgical resection. Although the role of surgery has not been validated through randomized trials, the favourable results reported in surgical series and the relative infrequency of long-term survival in patients treated without surgery established surgery as the treatment of choice.

Full Table

Full Table
The complete removal of the Stage I and II disease ensures a 5-year survival of about 60-80%.
By whom and where will stage I and II SCLC be coped with?
There is a worldwide scientific unanimity, saying that patients with Stage I and II NSCLC should be coped with, by specialized thoracic surgeons in specialized centers.
The specialization ensures a better outcome. It is emphatically considered that thoracic surgeons that exclusively perform thoracic surgeries at a 75% live better.
According to a study by Goodney et al. in 2005, out of 25,545 patients that were submitted to lobectomy or pneumonectomy for lung cancer, patients that were operated in hospitals that applied more than >20 thoracic surgeries a year had a mortality rate of 5.1% if the operation was made by a thoracic surgeon and 6.1% if the operation was made by a general surgeon. Also, in hospitals that had more than >45 thoracic surgeries a year, there was a 5% mortality rate if the operation was made by a thoracic surgeon and 6.1% if the operation was made by a general surgeon (P<0.01) (7).
Besides, a 2009 Farjah et al. study, that included 19,745 patients, showed that patients with lung cancer that were operated by general thoracic surgeons had an 11% lower death risk, as opposed to patients that were operated on by general surgeons (8).
In 2011, Ellis et al. reviewed 222,233 patients that were submitted to thoracic surgeries for lung cancer. The general thoracic surgeons (realized thoracic surgeries in more than 75%) performed better in lymphatic cleansing, morbidity and mortality than general surgeons (realized thoracic surgeries in less than 75%). Ellis et al. concluded that surgeon specialty impacts the adequacy of oncologic staging, in patients undergoing resection for primary lung cancer. Specifically, general thoracic surgeons performed intraoperative oncologic staging significantly more often than their general surgeon and cardiac surgeon counterparts, while achieving significantly lower in-hospital mortality and complication rates (9).
During a systematic study-analysis of articles published from January 1st 1990 until January 20th 2011, concerning the significance of the amount of incidence and the specialization of surgeons that applied surgical treatment to patients with lung cancer, Von Meyenfeldt discovered that the hospital volume and surgeon specialty are important determinants of the outcome in lung cancer resections (10).
Besides, the Ferraris et al. 2012 study concluded that patients operated on by thoracic surgeons have higher acuity compared to patients operated on by general surgeons. When patients are matched for comorbidities and serious preoperative risk factors, thoracic surgeons have improved outcomes, especially with regard to infectious complications and composite morbidity (11).
Studies have found that in cases involving thoracic surgeons, there is a lower operative mortality and morbidity, improved long-term survival, better adherence to established practice standards, and a lower cost compared with cases involving general surgeons. Some specific processes of care that account for these improved economic, operative, and oncological outcomes have been identified. Others are not yet specifically known and associated with specialization in thoracic surgery (12).
In 2001, Bach et al. analyzed the results of 2,118 lung cancer patients that were operated on, in 76 USA hospitals and concluded that patients who undergo resection for lung cancer at hospitals that perform large numbers of such procedures are likely to have got complication rates that were twice as high at hospitals with the lowest volume and to survive longer than patients who have such surgery at hospitals with a low volume of lung-resection procedures (13).
Meguid et al. studied 46,951 operations on lung cancer patients and proved that in-hospital mortality is reduced for patients undergoing lung cancer resections at teaching hospitals versus non-teaching hospitals (3.2% vs. 4.0%; P<0.001), with results prominent at all but the highest volume institutions (14).
However Kozower BD and Stukenborg GJ that in 2011, studied 4,460 patients from 436 hospitals, claim that hospital lung cancer resection volume is not a predictor of mortality and should not be used as a proxy measure for surgical quality (15).
Furthermore, in an interesting study by Dimick JB and Welch HG, it is proven that paradoxically, hospitals with a history of zero mortality subsequently experience mortality rates that are the same or higher than those of other hospitals. And in conclusion, the patients considering surgery should not consider a reported mortality of zero as being a reliable indicator of future performance but to take suspicion of the overall performance of the centre (16).
It therefore qualifies as a general view, that patients with οperable lung cancer should undergo surgical resection in organized centers with suitable care system and increased volume of cases by qualified and trained thoracic surgeons.
What is the ideal surgery for stage I and II NSCLC?
Surgery is the standard treatment for patients with clinical stage I and II NSCLC, in which there is no evidence of mediastinal involvement prior to surgical resection. Lobectomy, the surgical resection of a single lobe, is generally accepted as the optimal procedure for early stage NSCLC, because of its ability to preserve pulmonary function (17). In patients with early stage NSCLC, video-assisted thoracoscopic surgery (VATS) may be an alternative to open thoracotomy for patients undergoing lobectomy (18).
Indeed, according to Paul et al., which studied 6,323 patients that underwent surgical removal for early stage NSCLC from 2002 until 2007, 5,042 of which open thoracotomy and 1,281 VATS, patients that underwent VATS had less complications, fewer blood losses and consequently needed less transfusions, lower arrhythmia percentage and reintubation, the drainage tube was removed earlier and the hospitalization was shorter, while mortality was the same. The writers conclude that with correctly chosen patients, thoracoscopic lobectomy is preferred to the open (19). However, the study pointed out the smaller duration of the VATS lobectomy, with an average of 173 min as opposed to 143 min.
Also, in 2012 while Park et al. were studying 6,292 patients, out of which 1,523 had VATS lobectomy and 4,769 standard lobectomy, they found out that VATS had fewer complications (38% vs. 44%, P<0.001) and median LOS (5 vs. 7 days; P<0.001). Additionally, the patients undergoing VATS at high-volume VATS hospitals had shorter median LOS (4 vs. 6 days, P=0.001) compared with low-volume VATS hospitals (20).
In 2000 Sugi et al. studied the prognosis of 100 patients with NSCLC stage T1N0M0 out of which 52 were submitted to standard lobectomy and 48 to VATS. The writers concluded that VATS lobectomy with lymph node dissection achieved an excellent 5-year survival, similar to that achieved by the conventional approach. (The overall survival rates 5 years after surgery were 85% and 90% in the open and VATS groups, respectively) (21).
In 2007 De L Stanbridge et al. described an anterior minimally invasive thoracotomy with video assistance and found that this technique of using small anterior minithoracotomy for direct visualization or endoscopic visualization allowed the majority (82%) of patients with apotentially resectable lung cancer at any stage to have a surgically safe lung resection and showed no difference in survival when compared with open approaches (22).
Although it is admitted that nodal staging of NSCLC should be as accurate as possible, the extent of mediastinal lymph node assessment during surgery is controversial and there is no consensus.
There are different techniques used, ranging from simple visual inspection of the unopened mediastinum to an extended bilateral lymph node dissection. Furthermore, there are different terms used to define these techniques.
There are data which clearly show that systematic sampling or nodal dissection improves intraoperative staging.
The interesting article by Didier Lardinois et al. underlined that in the procedure of selected lymph node biopsy one or multiple suspicious lymph node(s) are biopsied. This is only justified to prove N1 or N2 disease in patients in whom resection is not possible (exploratory thoracotomy) (23).
Sampling is the removal of one or more lymph nodes guided by preoperative or intraoperative findings which are thought to be representative. Systematic sampling means a predetermined selection of the lymph node stations specified by the surgeon.
In systematic nodal dissection all the mediastinal tissue containing the lymph nodes is dissected and removed systematically within anatomical landmarks. For left-sided tumors, in order to get access to the high and low paratracheal nodes, the division of the ligamentum arteriosus can be added, resulting in the mobilization of the aortic arch. It was recommended that at least three mediastinal nodal stations (but always subcarinal) should be excised as a minimum requirement. The nodes are separately labeled and examined histologically. Beside the mediastinal nodes, the hilar and the intrapulmonary lymph nodes are dissected as well (24).
In lobe-specific systematic node dissection the mediastinal tissue containing specific lymph node stations is excised, depending on the lobar location of the primary tumor.
And finally in extended lymph node dissection bilateral mediastinal and cervical lymph node dissection is performed through median sternotomy and cervicotomy.
According to the findings of two randomized studies by Izbicki [2008] and Darling [2011], the mediastinal lymph node dissection (MLND) does not offer a survival advantage for resected stage I NSCLC (25,26).
Additionally a third randomized trial by Takizawa and al. comparing MLND and selective sampling did show that mediastinal lymph node sampling has got the similar effect to systematic nodal dissection in patients with clinical stage I NSCLC (27).
In the American College of Surgeons Oncology Group (ACOSOG) Z0030 trial by Allen et al. demonstrated that complete mediastinal lymphadenectomy adds little morbidity to a pulmonary resection for lung cancer (28).
As noted in the work of Didier Lardinois et al., the extended lymph node dissection of the mediastinum may be associated with excessive morbidity but according to the study of Naruke et al. in patients who had undergone radical operations--pulmonary resection combined with complete or extended MLND there was a significant difference between the prognosis for patients who had metastases to the subcarinal lymph nodes as compared to the prognosis for those who did not. The 5 years survival rates were 9.1% and 29.0%, respectively. In conclusion the procedure can be justified because of a survival benefit (29).
Smaller operations in surgical treatment of stage I and II NSCLC
The standard operation in surgical treatment for lung cancer has been lobectomy with systematic lymph node sampling or mediastinal node dissection. After lobectomy, patients with T1 N0 NSCLC experience up to an 80% 5-year cancer-free survival (30).In an attempt to preserve pulmonary function, in 1973 Jensik and colleagues were the first to suggest that a lesser resection—sublobar, might be an adequate operation for this stage of disease (31). However, the study of Robert J. Ginsberg and Lawrence V. Rubinstein in 1995 was catalytic. They designed a prospective, multiinstitutional randomized trial to compare limited resection with lobectomy for patients with peripheral T1 N0 NSCLC documented at operation. Analysis included locoregional and distant recurrence rates, 5-year survival rates, perioperative morbidity and mortality, and late pulmonary function assessment.
In patients undergoing limited resection locoregional recurrence rates was three times higher (5.4% vs. 1.9%, per patient per year). Overall survival was worse for sublobar resection (5-year survival 56% vs. 73%) but death from cancer was not statistically significant (26% vs. 19% for sublobar resection and lobectomy respectively).
Finally, they demonstrated that limited pulmonary resection does not confer improved perioperative morbidity, mortality, or late postoperative pulmonary function. Because of the higher death rate and locoregional recurrence rate associated with limited resection, lobectomy still must be considered the surgical procedure of choice for patients with peripheral T1 N0 NSCLC (32).
Okada M et al. designed an interesting study to compare sublobar resection (segmentectomy or wedge resection) with lobar resection to test which one is the appropriate procedure for peripheral cT1N0M0 NSCLC of 2 cm or less. Median follow-up was more than 5 years. Disease-free and overall survivals were similar in both groups with 5-year survivals of 85.9% and 89.6% for the sublobar resection group and 83.4% and 89.1% for the lobar resection group, respectively. Multivariate analysis confirmed that the recurrence rate and prognosis associated with sublobar resection were not inferior to those obtained with lobar resection. Postoperative lung function was significantly better in patients who underwent sublobar resection.
The researchers concluded that sublobar resection should be considered as an alternative for stage Ia NSCLCs 2 cm or less, even in low-risk patients (33).
Koike et al. demonstrated that in patients with peripheral T1N0M0 NSCLC whose maximum tumor diameter was 2 cm or less, the outcome of limited pulmonary resection is comparable with that of pulmonary lobectomy. 5-year survival 89.1% vs. 90.1% (34).
El-Sherif et al. compared the outcomes of all stage I NSCLC patients undergoing resection from 1990 to 2003. Lobectomy (577 patients) was the standard of care for patients with adequate cardiopulmonary reserve. Sublobar resection (207 patients) was reserved for patients with cardiopulmonary impairment prohibiting lobectomy. Compared with lobectomy, sublobar resection had no significant impact on disease-free survival (35).
Kates et al. tried to compare survival after lobectomy and limited resection among patients with stage Ia tumors ≤1 cm by using a large, US-based cancer registry. They identified 2,090 patients with stage I NSCLC ≤1 cm in size who underwent lobectomy or limited resection. They ultimately concluded that limited resection and lobectomy may lead to equivalent survival rates among patients with stage I NSCLC tumors ≤1 cm in size (36).
Wisnivesky et al. using the Surveillance, Epidemiology, and End Results registry, linked to Medicare records, identified 1,165 cases of stage I lung cancer < or =2 cm in size that underwent lobectomy or limited resection. The researchers found that survival of patients >65 years of age undergoing limited resection or lobectomy for stage Ia tumors < or =2 cm appears to be similar and conclude that limited resection may be an effective therapeutic alternative for these patients (37).
Some studies have shown low rates of surgical treatment of elderly patients with lung cancer due to morbidity and mortality.
However, the reported perioperative mortality of patients undergoing lobectomy with thoracotomy are 2% for age <60 years, 5% for age 60 to 69 years, 6% for age 70 to 79 years and 8% for age >80 years.
Moreover, perioperative mortality of patients undergoing pneumonectomy with thoracotomy is 7% for age <70 years, 16% for age 70 to 79 years and 28% for age >80 years (38).
But in a Mery et al. study with 14,555 patients it was proved that the median survival times were 71, 47, and 28 months, respectively, for patients <65, 65 to 74, and > or =75 years of age (P<0.0001). For the young patients, lobectomies conferred better survival times than limited resections. However, the statistical difference in long-term survival between those patients undergoing lobectomies and those undergoing limited resections disappeared at 71 years (39).
Dominguez-Ventura et al. studied the predictors of morbidity and mortality after pulmonary resection for lung cancer in 379 patients 80 years of age or older. Lobectomy had a higher risk of preoperative complication versus sublobar operation (51% vs. 36%) but 30-day mortality was not statistically significant (5% vs. 8.4%) for lobectomy and sublobar resection respectively. The overall operative mortality was 6.3% and significant predictors were congestive heart failure and prior myocardial infarction. Factors not associated with mortality included previous myocardial revascularization, renal insufficiency (creatinine >1.5 mg/dL), and diabetes mellitus (40).
All these data showed that sublobar resection in appropriate selected patients, result in reasonable outcomes. But clearly notes that the general condition of the patient dictates decisions and affects the type of surgery that was finally progressing and not chronological age.
Which of the smallest operations in surgical treatment of stage I and II NSCLC, surpasses, segmentectomy or wedge resection?
Undoubtedly the segmentectomy has the advantage of complete ablation of the vascular and lymphatic drainage of the primary tumor but also appears to provide better parenchymal resection limits.
Sienel et al. decided to analyze the cancer-related survival of the wedge resection with systematic lymphadenectomy and the segmentectomy with systematic lymphadenectomy in patients with stage I lung cancer. There were significantly less locoregional recurrences and a better cancer-related survival following segmentectomies compared to wedge resections. Therefore if limited functional operability requires a sublobar resection for a patient of stage I NSCLC, the authors conclude that it is preferable segmental with systematic lymphadenectomy resection of the wedge (41).
Nakamura et al. compared the surgical outcomes of lobectomy, segmentectomy and wedge resection by VATS for clinical stage I NSCLC, retrospectively. The 5-year survival rates for the lobectomy, segmentectomy, and wedge resection groups were 82.1%, 87.2%, and 55.4%, respectively (42). These and other data suggest strongly that wedge resection for clinical stage I NSCLC should be carefully indicated and requires adequate patient selection.
What are the safest, ideal margins of excision? What are the safest, ideal margins of resection?
Τhere are few studies that determine safe margins of excision. In 2000 Kara et al. showed that a bronchial resection margin of 1.5 cm in length from the macroscopic tumor will provide tumor-free margins in 93% of NSCLC cases. The Authors found that Adenocarcinoma showed more peribronchial extension (80.0%) whereas squamous cell carcinoma (63.6%) showed more bronchial extension (43).
This rule overturned the findings of Tomaszek. Tomaszek et al. studied 3,936 consecutive pulmonary resections that were performed between 1992 and 2007 at Mayo Clinic and showed that when complete surgical resection is achieved, the extent of the bronchial margin has no clinically relevant impact on disease-free and overall survival in early-stage non-small-cell lung cancer. Therefore, they demonstrated that R0 resection is the best treatment (44).
Sawabata et al. in 2004 showed that the rate of malignant negative margins was 100% when the margin distance was greater than 20 mm, and the rate of malignant negative margins was 100% when the resected tumors had a margin distance greater than the maximum tumor diameter (45).
According El-Sherif et al. margin is an important consideration after sublobar resection of NSCLC. Wedge resection is frequently associated with margins less than 1 cm and a high risk for locoregional recurrence (46). In conclusion sufficient and safe margin of sublobar resection is considered of 2 cm.
Therefore, the best treatment of patients with stage I or II lung cancer are lobectomy with systematic lymphadenectomy, which is preferable to apply VATS in specialized centers with experience and knowledge.
Sublobar resection (segmentectomy versus wedge resection) is recommended for patients with decreased pulmonary function who may tolerate operative intervention.
Necessary to achieve clear margins above the maximum tumor diameter for lesions less than 2 cm and limits at least 2 cm for tumors greater than 2 cm.
Surgical treatment of the tumors invading chest wall (tumor T3)
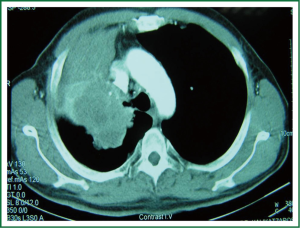
Stoelben and Ludwig demonstrate that lung cancer invasion of the chest wall is not a common challenge and represents only about 5% of all patients resected for lung cancer and that in T3N0M0 tumours long-term survival reaches 40-50% (47) (Figure 1).
Magdeleinat et al., found that the completeness of resection, nodal involvement, depth of invasion, and age affect the survival of patients with lung cancer invading the chest wall. The authors believe that N2 disease should not be considered a contraindication to surgery because it seems that the actuarial 5-year survival after complete resection was 21% in T3N2 disease (48).
Sanli et al. and Deslauriers et al., reported that the most important factors affecting the survival in both T3 and T4 tumors (vertebra) as well as Pancoast tumors are the complete resection application and the pathologic nodal status (49,50).
In their study on 107 patients who underwent surgical resection for chest wall invading NSCLC, Lee et al. agree that the completeness of resection, nodal status, depth of invasion, tumor size, and adjuvant chemotherapy were prognostic factors for long-term survival. Overall 5-year survival rate was 26.3% (51).
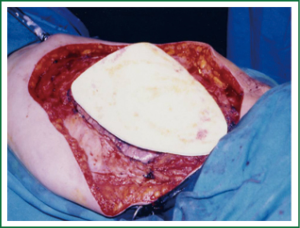
The best materials for the restoration of the chest wall are: methyl metacrylate, polytetrafluoroethylene (PTFE), metallic rods and plates (52,53) (Figure 2).
Miller et al., reconstructed the chest wall with biomaterials. Their early results are promising. Biomaterials may be the preferred method of reconstruction for infected chest wall sites (54).
Berry et al., describe a new hybrid technique where thoracoscopic techniques were utilized to accomplish the pulmonary resection and a limited counter incision was used to perform the en-bloc resection of the chest wall, avoiding scapular mobilization and rib spreading (55).
Cerfolio et al., within the last years, used a new technique that avoids cutting of the extrathoracic (trapezius, rhomboids, serratus anterior) muscles. Ribs with invading cancer are resected from inside of the chest instead of cutting the uninvolved muscles over them. The approach used, can be a thoracotomy, robotic, or video-assisted technique (56).
Burkhart et al., in their work on 95 patients with lung cancer invading the chest wall who underwent en bloc lung and chest wall resection found that this operation was safe but associated with significant morbidity. The best survival is observed in women who have T3N0M0 disease (stage IIB) (57).
Surgical treatment of the tumors invading diaphragm (tumor T3)
Invasion of the diaphragm is classified T3 in the new 7th TNM classification.
Weksler et al. studied from January 31, 1974, to August 17, 1995, a total of 4,668 patients that underwent exploration for resection of NSCLC at Memorial Hospital. By analyzing the database they identified eight patients (0.17%) who had exploratory thoracotomy for resection of NSCLC invading the diaphragm. The authors believe that patients without involved mediastinal nodes and in good general condition, diaphragmatic invasion should be treated by resecting the tumor “en bloc’’ with the diaphragm (58). Rocco et al. reported that T3 lung cancer disease invading the diaphragm is best treated with en bloc resections with wide tumor-free margins and prosthetic replacement of the diaphragm. The actuarial 5-year survival was 20% (59).
However, Yokoi et al. in their study on 63 patients who underwent resection of T3 lung cancer invading the diaphragm concluded that in selected patients with lung carcinoma and diaphragmatic invasion, combined resection of the lung and diaphragm offers the prospect of cure with acceptable mortality. The authors believe that primary lung tumors with diaphragmatic invasion, especially invasion of the muscle layer or deeper tissue, are not considered to be T3 lesions, because these cancers are generally technically resectable but oncologically almost incurable (60).
Survival is better, in patients with T3N0M0 disease, as well as with lung cancer invading the chest wall. Wide margins should be achieved. Direct primary repair of the diaphragm is possible in patients with limited invasion. In those with extensive involvement, complete resection and reconstruction of the diaphragm with a mesh (PTFE) is recommended (61).
Surgical treatment of pancoast tumors (superior sulcus tumors-T3 disease)
Non-small-cell lung carcinomas of the superior sulcus, frequently termed Pancoast tumours, are some of the most challenging thoracic malignant diseases to treat because of their proximity to vital structures at the thoracic inlet. Originally deemed universally fatal, Pancoast tumours are now amenable to curative treatment because of improvements in combined modality therapy and development of new techniques for resection.
Kappers et al. reported that the combination of radiotherapy and concurrent chemotherapy followed by surgery (trimodality treatment) is currently regarded as optimal treatment for NSCLC of the superior sulcus or Pancoast tumour. The 2- and 5-year survival after induction treatment and surgery was 75% and 39%, respectively. Local recurrence rates were 0% after induction treatment and surgical resection, 32% after concurrent chemoradiotherapy and 72% after (sequential) radiotherapy and/or chemotherapy (62).
Kunitoh et al., concluded that this trimodality therapy (chemotherapy, radiotherapy thoracotomy) is safe and effective for the treatment of patients with superior sulcus tumors (63).
Yildizeli et al. recorded a successful outcome on 126 patients with superior sulcus tumors who underwent surgical treatment. Overall 5-year survival rate was 36.6% (64).
In their study, Demir et al. investigated the treatment modalities and factors influencing survival in surgically treated superior sulcus tumors and concluded that the presence of N2 disease and incomplete resection are the two most important factors affecting survival while induction chemotherapy/radiotherapy may increase the ability to achieve complete surgical resection (65).
Vos et al., report their experience on 54 patients that were treated by chemotherapy (cisplatin/etoposide) and concurrent radiotherapy (46-66 Gy) followed by surgical resection. A complete (R0) resection was performed in 82% of 54 patients and 2-year survival was 50% (66).
In conclusion, pancoast tumors (superior sulcus tumors or apical lung tumors) typically invade structures at the thoracic outlet, including the inferior elements of the brachial plexus (C8, T1 nerve roots and lower trunk). Historically, these tumors are rapidly fatal, but newer treatment with induction chemotherapy and radiotherapy, followed by surgical resection of the tumor has resulted in improved patient survival (67).
Surgical treatment of the patients with stage III NSCLC
Historically, stage III lung cancer was defined as locoregionally advanced disease attributed to primary tumor extension into extrapulmonary structures or mediastinal lymph node involvement without evidence of distant metastases. Stage IIIa disease [T1, T2N2M0, T3N1, N2M0, T4 (two nodules in the ipsilateral lung, extension to adjacent organs) N0, N1, M0].
We have accepted the division of this patient group into three main subgroups:
- Patients disclosed intraoperative N2 disease despite thorough preoperative staging;
- Patients with preoperative evidence of N2 disease based on the findings of CT or CT-PET;
- Patients with disease apparently invaded the lymph nodes of the mediastinum (N2 involvement), bulky disease.
Patients with occult N2 disease despite thorough preoperative tests
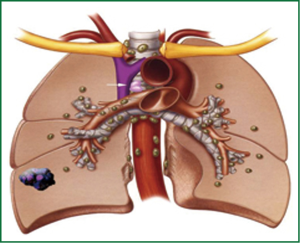
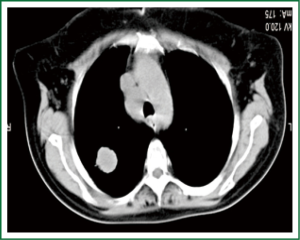
In this group of patients shows “unexpected” mediastinal nodal involvement intraoperative (Figures 3,4).
Goldstraw et al., in an interesting study which included 876 patients operated with NSCLC concluded that despite rigorous preoperative investigations, routine mediastinal node dissection demonstrated mediastinal node metastasis for the first time at thoracotomy in 26% of their patients (68).
Cerfolio et al., observed that in over 137 patients staged as N0 using PET/CT and CT, only 4.7% had N2 disease after surgery (69).
Al-Sarraf et al., in their study in 2008, found that over 215 patients with NSCLC who are clinically staged as N2/N3 negative in the mediastinum by integrated PET-CT, 16% will have occult N2 disease following resection (70).
Therefore even the diligent preoperative staging with modern testing shows that the true “unexpected” positive N2 disease is about 16%.
Allen et al., in their study with 1,023 patients, concluded that there is no significant statistical difference on morbidity and mortality of patients undergoing lymph node sampling or lymph node dissection (71).
The role of primary surgery in the management of occult N2 disease, “unexpected” mediastinal nodal involvement showed intraoperative, is important.
The surgery should be continued, with a lobectomy performed and MLND. Many surgeons have serious reservations whether the radical resection of the disease requires pneumonectomy. The overall 5-year survival rate of the patients with N2 disease identified, “unexpectedly”, at thoracotomy who undergoing complete and effective surgical resection is 30%. This is the conclusion of Rusch in their work published in 1996 (72).
However, it is important to emphasize that the proportion of patients in whom complete resection (R0 resection) is not achieved is about 35% for N2 disease (73).
Some authors claim the cancellation of surgery if complete resection is impossible since the 5-year survival is <5% (74).
Patients with preoperative evidence or suspected N2 disease
First of all, patients in whom the possibility of N2 involvement is suspected must undergo a careful staging evaluation (75).
A 2007 Cochrane meta-analysis by Burdett et al. about the role of pre-operative chemotherapy in the treatment of patients with non-small cell lung staging I-III showed that pre-operative chemotherapy increased survival with a hazard ratio of 0.82 (95% CI: 0.69-0.97) P=0.022 (76).
Similarly in a previous study when this meta-analysis limited to only patients with stage III disease a 0.73 (95% CI: 0.51-1.07; P=0.1) hazard ratio was found.
Additionally, in their work Gilligan et al. researched whether patients with operable NSCLC of any stage, outcomes could be improved by giving platinum-based chemotherapy before surgery. Finally the authors concluded that there was no evidence of a difference in overall survival with neo-adjuvant chemotherapy (77).
Therefore, thorough analysis of these data demonstrates that patients with N2 disease identified preoperatively (or suspected) to be treated more favorably with neoadjuvant chemotherapy followed radical resection.
However, many authors have been talking about whether there are certain subsets of patients with N2 disease in whom primary surgery is the best treatment. It seems that there are no safe criteria for the selection of patients.
Patients with bulky (unresectable) disease
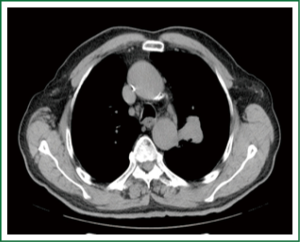
Patients with extensive disease (bulky) are treated with a combination of chemotherapy and radiotherapy. However, for this group of patients the optimal therapy has not been determined yet (78) (Figures 5,6).
Treatment of patients with NSCLC stage IIIB disease
Unfortunately, most patients with lung cancer are diagnosed when the cancer is already advanced (stage IIIB or IV), and they are no longer candidates for surgical resection (79). At stage IIIB, classified patients with tumors T4N2M0 and any TN3M0. N3 lung cancer disease means: Metastasis in the contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph nodes. T4 lung cancer disease means: tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, or carina; or separate tumor nodule(s) in a different ipsilateral lobe. These patients are considered inoperable (80).
Lung cancer surgery with solitary brain metastasis
Brain metastases occur in 30% to 50% of patients with NSCLC and confer a worse prognosis and quality of life (81). Some patients with resectable NSCLC have simultaneously solitary brain metastasis. This specialized group of patients is treated with removal of solitary brain metastasis followed by surgical resection of the primary tumor of the lung. Read et al., found that the survival rate after curative lung and brain resection was 21% at 5 years (82).
It is known by the work of Patchell et al., who showed that patients with cancer and a single metastasis to the brain who receive treatment with surgical resection plus radiotherapy live longer, have fewer recurrences of cancer in the brain, and have a better quality of life than similar patients treated with radiotherapy alone (83).
Burt et al., on 185 patients undergoing resection of brain metastases from NSCLC recorded the overall survival rates were as follows: 1 year, 55%; 2 years, 27%; 3 years, 18%; 5 years, 13%; and 10 years, 7% (median 14 months) (84).
Trillet et al. note that patients treated with surgery have a better survival (median 10 vs. 4.5 months) than the others, and among surgically treated patients only those treated with bifocal resection are long-term survivors (85).
Billing et al., in their work with 220 patients that underwent surgical treatment for brain metastases from NSCLC, argues that although the overall survival for patients who have brain metastases from NSCLC is poor, surgical resection may prove beneficial in a select group of patients with synchronous brain metastases and lung cancer without lymph node metastases with survival at 5 years about 21% (86).
Hu et al., reached to an interesting conclusion according to which the thoracic stage I NSCLC patients with solitary brain metastases in their study had a more favourable outcome than would be expected and was comparable to stage I NSCLC without brain metastases (87).
Lo et al., reported their surgical experience on 17 patients with synchronous primary lung cancer and solitary brain metastasis treated by pulmonary resection and neurosurgical intervention with a 5-year survival of about 27% (88).
Villarreal-Garza et al., in their study reviewed published series of patients with NSCLC and with brain metastases treated with aggressive thoracic management, with either lung tumour resection or thoracic radiation with or without chemotherapy as a definitive treatment. Patients treated with aggressive radiotherapy with or without chemotherapy, achieved a 2-year survival of 16-60%. Patients treated with surgical resection for the primary lung tumour, achieved a 5-year survival by 11-24% (89).
Today solitary brain metastases are treated with surgery. Craniotomy is performed first, followed by thoracotomy shortly (2-3 weeks) after. The overall 5-year survival for patients with NSCLC and solitary brain metastases may reach up to 25%.
Lung cancer surgery with solitary adrenal metastasis
In the 1980s several cases of simultaneous resection of NSCLC and adrenal metastasis with long-term survival were reported. However, first Porte et al., suggested the resection of synchronous lesions, solitary adrenal gland metastases, in 11 patients with operable NSCLC without N2 involvement with no additional mortality or morbidity (90). In 2001 the same author and coauthors confirmed the possibility of long-term survival after resection of isolated adrenal metastasis from NSCLC on 11 patients. Median survival was 11 months and 3 patients survived more than 5 years (91).
Pfannschmidt et al., on 11 patients that underwent curative resection for metastatic NSCLC of the adrenal gland concluded that adrenalectomy for clinically solitary, resectable metastases can be safely performed (92).
Sebag et al., emphasized on the benefits of a laparoscopic approach of tumor resection (93).
Mordant et al. found that NSCLC patients with synchronous solitary adrenal may benefit from lung resection on a curative intend in the case of adenocarcinoma and N0 extension whose complete resection is achievable with a lobectomy (94).
Kozower et al., believed that highly selected patients with a solitary focus of metastatic disease in the brain or adrenal gland appear to benefit from resection or stereotactic radiosurgery. This is particularly true in patients with a long disease-free interval (95).
Griffioen et al., in a recent work argue strongly that radical treatment of selected NSCLC patients presenting with 1-3 synchronous metastases, can result in favorable 2-year survivals (96).
Surgery in SCLC
SCLC is considered a systemic disease at diagnosis, because the potential for hematogenous and lymphogenic metastases is very high. For many years, the diagnosis of SCLC was considered a contraindication for surgery because radiotherapy was at least equivalent in terms of local control, and the rate of resectability in SCLC patients was poor. When chemotherapy became the mainstay of treatment for SCLC, radiotherapy was its logical complement, and surgery was progressively abandoned.
However, the role of surgical intervention in the multimodality management of SCLC continues to be controversial. At most, only 5-8% of patients with this disease can be considered initially as potential surgical candidates. These are patients who can be clinically classified as having stage I, II or resectable stage IIIa disease, as defined by the International TNM. This small group of patients comprises 15-25% of patients with limited disease. Actually, this number is even smaller, if one excludes patients with N2 disease.
The efficacy of surgical intervention for SCLC there is not clear.
Yu et al., studied the clinical outcomes of patients undergoing surgical treatment for stage I SCLC from 1988 to 2004.They concluded that surgical intervention seems to offer reasonable overall survival results in a cohort of stage I patients who undergo lobectomy (97).
Koizumi T et al., between January 1991 and December 2010, thoracotomy was performed in a total of 3,776 cases of primary lung cancer. Among them, 69 cases of SCLC. In these lobectomy was performed in 53 patients, pneumonectomy was performed in 3 patients and segmentectomy or partial resection was performed in 13 patients.
According to the pathological stages in patients with resected SCLC, the 5-year survival rate was 43.1%, in stage I, 37.8% in stage II, and 17.7% in stage III, respectively.
Authors of the study showed that the clinical outcomes in patients initially underwent surgical resection for SCLC, are favourable and demonstrated a 5-year survival rate of 34.3% (98).
Chandra et al., reported an overall 5-year survival rate of 27% on 67 patients, who underwent thoracotomy for SCLC (99).
Lim et al. recently described good results at a 5-year survival rate of 52% for patients with limited disease SCLC stage I to III, who underwent lung resection (100).
In conclusion, many interesting studies agree that the indications for surgical interventions for SCLC are:
- Achieving local control of the disease;
- Treatment for tumours with mixed histology (SCLC and adenocarcinoma of the lung);
- In cases without lymph node metastasis after nodal evaluation using diagnostic imaging such as PET-CT, and mediastinoscopy or EBUS-TBNA (101-103).
Advances in surgical treatment of lung cancer
Treatment of lung cancer is progressing rapidly, with significant advances in all modalities, including surgery, radiation, and chemotherapy. Although the best therapeutic approach for NSCLC is a multimodality therapy, surgical removal remains the cornerstone for early stage carcinomas (104). Lung cancer resection can be performed using several surgical techniques. Location, number and extension of surgical incisions, total or partial muscle sparring techniques, VATS and the use of robotic devices for camera holding or fine vascular and lymphatic dissections are some of the variables considered when planning lung cancer resection (105). VATS lobectomy is a safe, efficient, well accepted and widespread technique among thoracic surgeons, but standard VATS forceps have rigid extremities and do not mimic wrist angulated movements. Furthermore, traditional VATS video-imaging is a simple two dimensional image (106). Robotic surgery is performed with telemanipulated flexible effector instruments; some of which can give surgeons tactile feedback; and under three-dimensional (3-D) video-imaging. Hilar pulmonary dissection for lung cancer can be performed by robotic devices in an efficient and safe way (107,108).
More indications for thoracoscopic treatment of lung cancer
Thoracoscopic lobectomy is well established for the treatment of early NSCLC. Its safety and efficacy for advanced-stage disease remain uncertain. Hennon and colleagues in 2011 found that thoracoscopic lobectomy for advanced-stage NSCLC can be performed safely, with results equivalent to open techniques. In their study on 125 patients there were no differences between the thoracoscopic and open groups in overall survival (43.7 vs. 22.9 months; P=0.59) and disease-free survival (34.7 vs. 16.7 months; P=0.84) (109). Although the effectiveness of surgical assisted thoracoscopy regarding disputed radicalness of lymphadenectomy but newer techniques arise which facilitate the treatment of patients with lung cancer. (Fluoroscopy assisted thoracoscopic resection, Video-assisted radiofrequency ablation, Single-Incision Thoracoscopy etc.) (110-113). The limits of thoracoscopic resections are expanding, with improved instruments for manipulating and dividing tissues such as the bone. So the removal of the chest wall can be applied with thoracoscopy (114).
Wu applied thoracoscopic lobectomy in 36 patients using anaesthesia without tracheal intubation (using epidural anaesthesia, intrathoracic vagal blockade, and sedation) and showed that non intubated thoracoscopic lobectomy is technically feasible and was as safe as thoracoscopic lobectomy performed with tracheal intubation in the geriatric lung cancer patients (115). To minimize this damage, Oda et al., used total port-access, video-assisted thoracoscopic lobectomy via the subcostal trans-diaphragmatic approach by using three 5-mm intercostal ports and one 15-mm subcostal trans-diaphragmatic port for endostaplers and instruments >5 mm in diameter. The researchers believe that this approach is feasible and safe, easy for experienced VATS surgeons to learn, and has the potential advantage of minimizing postoperative pain (116). Akiba et al., demonstrate the utility of SECUREA, a polyurethane sponge with a radiopaque marker, for complete thoracoscopic MLND in patients with NSCLC (117).
In conclusion, VATS is emerging as a therapeutic option for a variety of thoracic applications. When applied to the patient with lung cancer, the therapeutic benefit of VATS lobectomy appears to be confined to node-negative, relatively small tumours. Operable patients with larger tumours are currently best served by thoracotomy and MLND. As an alternative to thoracotomy for stage I lung cancer, VATS lobectomy is associated with less postoperative pain, less surgical morbidity, fewer complications, and shorter hospitalization. Additionally, improved technology and instrumentation now allow for equivalent and sometimes superior surgical retraction and exposure that can mimic that of an open operation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;15:2893-917. [PubMed]
- Goldstraw P. eds. IASLC staging manual in thoracic oncology. FL: Editorial Rx Press, 2009.
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind Ch. eds. International union against cancer, tnm classification of malignant tumours. 7th edition. Oxford: Wiley-Blackwell, 2010.
- Edge SB, Byrd DR, Compton CC, et al. eds. American Joint Committee on Cancer, Cancer Staging Manual. 7th edition. Springer: New York, 2010:254-70.
- Goldstraw P, Crowley JJ. The International Association for the Study of Lung Cancer international staging project on lung cancer. J Thorac Oncol 2006;1:281-6.
- Goodney PP, Lucas FL, Stukel TA, et al. Surgeon specialty and operative mortality with lung resection. Ann Surg 2005;241:179-84. [PubMed]
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004; discussion 1005-6. [PubMed]
- Ellis MC, Diggs BS, Vetto JT, et al. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg 2011;92:1958-63; discussion 1963-4.
- von Meyenfeldt EM, Gooiker GA, van Gijn W, et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol 2012;7:1170-8. [PubMed]
- Ferraris VA, Saha SP, Davenport DL, et al. Thoracic surgery in the real world: does surgical specialty affect outcomes in patients having general thoracic operations? Ann Thorac Surg 2012;93:1041-7. [PubMed]
- Tieu B, Schipper P. Specialty matters in the treatment of lung cancer. Semin Thorac Cardiovasc Surg 2012;24:99-105. [PubMed]
- Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181-8. [PubMed]
- Meguid RA, Brooke BS, Chang DC, et al. Are surgical outcomes for lung cancer resections improved at teaching hospitals? Ann Thorac Surg 2008;85:1015-24; discussion 1024-5. [PubMed]
- Kozower BD, Stukenborg GJ. The relationship between hospital lung cancer resection volume and patient mortality risk. Ann Surg 2011;254:1032-7. [PubMed]
- Dimick JB, Welch HG. The zero mortality paradox in surgery. J Am Coll Surg 2008;206:13-6. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1. [PubMed]
- De L. Minimally invasive anterior thoracotomy for routine lung cancer resection. Innovations (Phila) 2007;2:76-83. [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [PubMed]
- Goldstraw P. Report on the international workshop on intrathoracic staging. Lung Cancer 1997;18:107-11.
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Takizawa H, Kondo K, Matsuoka H, et al. Effect of mediastinal lymph nodes sampling in patients with clinical stage I non-small cell lung cancer. J Med Invest 2008;55:37-43. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [PubMed]
- Thomas P, Rubinstein L. Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1990;49:242-6; discussion 246-7. [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [PubMed]
- Kiser A. General aspects of surgical treatment. In: Detterbeck F, Rivera MP, Socinski MA, et al. eds. Diagnosis and treatment of lung cancer-an evidence based guide. Philadelphia: W B Saunders, 2001:177-90.
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006;82:1175-9. [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [PubMed]
- Kara M, Sak SD, Orhan D, et al. Changing patterns of lung cancer; (3/4 in.) 1.9 cm; still a safe length for bronchial resection margin? Lung Cancer 2000;30:161-8. [PubMed]
- Tomaszek SC, Kim Y, Cassivi SD, et al. Bronchial resection margin length and clinical outcome in non-small cell lung cancer. Eur J Cardiothorac Surg 2011;40:1151-6. [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [PubMed]
- Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg 2009;35:450-6. [PubMed]
- Magdeleinat P, Alifano M, Benbrahem C, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg 2001;71:1094-9. [PubMed]
- Sanli A, Onen A, Yücesoy K, et al. Surgical treatment in non-small cell lung cancer invading to the chest wall (T3) and vertebra (T4). Tuberk Toraks 2007;55:383-9. [PubMed]
- Deslauriers J, Tronc F, Fortin D. Management of tumors involving the chest wall including pancoast tumors and tumors invading the spine. Thorac Surg Clin 2013;23:313-25. [PubMed]
- Lee CY, Byun CS, Lee JG, et al. The prognostic factors of resected non-small cell lung cancer with chest wall invasion. World J Surg Oncol 2012;10:9. [PubMed]
- Doddoli C, D’Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [PubMed]
- Nakada T, Akiba T, Inagaki T, et al. A Rare Case of Primary Intercostal Leiomyoma: Complete Resection Followed by Reconstruction Using a Gore-Tex(®) Dual Mesh. Ann Thorac Cardiovasc Surg 2013. [Epub ahead of print].
- Miller DL, Force SD, Pickens A, et al. Chest wall reconstruction using biomaterials. Ann Thorac Surg 2013;95:1050-6. [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Minimally invasive chest wall resection: sparing the overlying, uninvolved extrathoracic musculature of the chest. Ann Thorac Surg 2012;94:1744-7. [PubMed]
- Burkhart HM, Allen MS, Nichols FC 3rd, et al. Results of en bloc resection for bronchogenic carcinoma with chest wall invasion. J Thorac Cardiovasc Surg 2002;123:670-5. [PubMed]
- Weksler B, Bains M, Burt M, et al. Resection of lung cancer invading the diaphragm. J Thorac Cardiovasc Surg 1997;114:500-1. [PubMed]
- Rocco G, Rendina EA, Meroni A, et al. Prognostic factors after surgical treatment of lung cancer invading the diaphragm. Ann Thorac Surg 1999;68:2065-8. [PubMed]
- Yokoi K, Tsuchiya R, Mori T, et al. Results of surgical treatment of lung cancer involving the diaphragm. J Thorac Cardiovasc Surg 2000;120:799-805. [PubMed]
- Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev 2013;22:382-404. [PubMed]
- Kappers I, Belderbos JS, Burgers JA, et al. Non-small cell lung carcinoma of the superior sulcus: favourable outcomes of combined modality treatment in carefully selected patients. Lung Cancer 2008;59:385-90. [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5. [PubMed]
- Demir A, Sayar A, Kocaturk CI, et al. Surgical treatment of superior sulcus tumors: results and prognostic factors. Thorac Cardiovasc Surg 2009;57:96-101. [PubMed]
- Vos CG, Hartemink KJ, Blaauwgeers JL, et al. Trimodality therapy for superior sulcus tumours: evolution and evaluation of a treatment protocol. Eur J Surg Oncol 2013;39:197-203. [PubMed]
- Ripley RT, Rusch VW. Role of induction therapy: surgical resection of non-small cell lung cancer after induction therapy. Thorac Surg Clin 2013;23:273-85. [PubMed]
- Goldstraw P, Mannam GC, Kaplan DK, et al. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). J Thorac Cardiovasc Surg 1994;107:19-27; discussion 27-8. [PubMed]
- Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest 2006;130:1791-5. [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Rusch VW. Surgical Treatment of Patients With N2 Disease. Semin Radiat Oncol 1996;6:76-85. [PubMed]
- Detterbeck F. What to do with “Surprise” N2?: intraoperative management of patients with non-small cell lung cancer. J Thorac Oncol 2008;3:289-302. [PubMed]
- Detterbeck FC, Jones DR. Surgical treatment of stage IIIa (N2) nonsmall cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, et al. eds. Diagnosis and Treatment of Lung Cancer: An Evidence-Based Guide for the Practicing Clinician. Philadelphia, PA: W. B. Saunders, 2001:244-56.
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev 2007;CD006157. [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [PubMed]
- Is there a role for surgery in stage IIIA-N2 non-small cell lung cancer? Zhongguo Fei Ai Za Zhi 2008;11:615-21. [PubMed]
- Maher AR, Miake-Lye IM, Beroes JM, et al. Treatment of metastatic non-small cell lung cancer: a systematic review of comparative effectiveness and cost-effectiveness. Washington (DC): Department of Veterans Affairs (US); 2012 Oct. VA Evidence-based Synthesis Program Reports.
- Sculier JP. Nonsmall cell lung cancer. Eur Respir Rev 2013;22:33-6. [PubMed]
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [PubMed]
- Read RC, Boop WC, Yoder G, et al. Management of nonsmall cell lung carcinoma with solitary brain metastasis. J Thorac Cardiovasc Surg 1989;98:884-90; discussion 890-1. [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [PubMed]
- Burt M, Wronski M, Arbit E, et al. Resection of brain metastases from non-small-cell lung carcinoma. Results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg 1992;103:399-410; discussion 410-1. [PubMed]
- Trillet V, Catajar JF, Croisile B, et al. Cerebral metastases as first symptom of bronchogenic carcinoma. A prospective study of 37 cases. Cancer 1991;67:2935-40. [PubMed]
- Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53. [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [PubMed]
- Lo CK, Yu CH, Ma CC, et al. Surgical management of primary non-small-cell carcinoma of lung with synchronous solitary brain metastasis: local experience. Hong Kong Med J 2010;16:186-91. [PubMed]
- Villarreal-Garza C, de la Mata D, Zavala DG, et al. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer 2013;14:6-13. [PubMed]
- Porte HL, Roumilhac D, Graziana JP, et al. Adrenalectomy for a solitary adrenal metastasis from lung cancer. Ann Thorac Surg 1998;65:331-5. [PubMed]
- Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg 2001;71:981-5. [PubMed]
- Pfannschmidt J, Schlolaut B, Muley T, et al. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer 2005;49:203-7. [PubMed]
- Sebag F, Calzolari F, Harding J, et al. Isolated adrenal metastasis: the role of laparoscopic surgery. World J Surg 2006;30:888-92. [PubMed]
- Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 2012;41:617-22. [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): Patient outcomes and prognostic factors. Lung Cancer 2013. [Epub ahead of print]. [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [PubMed]
- Koizumi T, Fukushima T, Hamanaka K, et al. Surgical outcomes in patients with small cell lung cancer: comparative analysis of computed tomograpy-detected patients with others. World J Surg Oncol 2013;11:61. [PubMed]
- Chandra V, Allen MS, Nichols FC 3rd, et al. The role of pulmonary resection in small cell lung cancer. Mayo Clin Proc 2006;81:619-24. [PubMed]
- Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267-71. [PubMed]
- Baltayiannis N, Bolanos N, Anagnostopoulos D, et al. Surgery in small cell lung cancer: when and why. J BUON 2005;10:459-72. [PubMed]
- Inoue M, Sawabata N, Okumura M. Surgical intervention for small-cell lung cancer: what is the surgical role? Gen Thorac Cardiovasc Surg 2012;60:401-5. [PubMed]
- Koletsis EN, Prokakis C, Karanikolas M, et al. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg 2009;4:30. [PubMed]
- Ikeda N, Saji H, Hagiwara M, et al. Recent advances in video-assisted thoracoscopic surgery for lung cancer. Asian J Endosc Surg 2013;6:9-13. [PubMed]
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [PubMed]
- Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg 1993;56:784-6. [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [PubMed]
- das-Neves-Pereira JC, Riquet M, Le Pimpec-Barthes F, et al. Robotic Surgery for Lung Cancer. In: Baik SH. eds. Robot Surgery 2012:172. INTECH, Croatia, downloaded from SCIYO.COM.135-148.
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph Node Evaluation Achieved by Open Lobectomy Compared With Thoracoscopic Lobectomy for N0 Lung Cancer. Ann Thorac Surg 2013. [Epub ahead of print]. [PubMed]
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [PubMed]
- Shen Y, Zhong M, Jiang W, et al. Video-assisted radiofrequency ablation for pleural disseminated non-small cell lung cancer. BMC Surg 2013;13:19. [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [PubMed]
- Hennon MW, Demmy TL. Thoracoscopic resection and re-resection of an anterior chest wall chondrosarcoma. Innovations (Phila) 2012;7:445-7. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [PubMed]
- Oda M, Matsumoto I, Waseda R, et al. Total port-access lobectomy via a subcostal trans-diaphragmatic approach for lung cancer. Interact Cardiovasc Thorac Surg 2013;16:211-3. [PubMed]
- Akiba T, Marushima H, Hirano K, et al. Thoracoscopic mediastinal lymph node dissection using an endoscopic spacer. Ann Thorac Cardiovasc Surg 2012;18:281-3. [PubMed]