Metastasectomy in pediatric patients: indications, technical tips and outcomes
Introduction
Lung metastases occur in 10–30% of pediatric solid tumors and their treatment plays an important role on disease control and long-term survival (1). As metastases are expression of a disseminated process, the outcomes depend mainly on effective systemic therapy but surgery has a therapeutic role in selected cases.
Actually, pulmonary metastasectomy has become a standard procedure for pediatric patients with certain types of solid tumors (2), being considered a technically feasible, safe and potentially curative treatment associated with low morbidity and mortality (3). The role of metastasectomy has been actively investigated in adult patients, increasing the 5 years survival from 10% to 40–55% (4).
Studies on children are less significant because of the rarity of pediatric solid neoplasms but surgery has been evaluated in the context of multidisciplinary approach. Surgeons, expert pediatric oncologists and radiation oncologists contribute with their different skills to the management of pulmonary metastases.
Surgical excision of lung deposits is nowadays an accepted treatment option for metastatic pediatric tumors. Starting from 1950s, single cases and few case-series experiences are reported. Case-series became numerically significant in 1970s and most of them included different types of tumors (5-7).
Since those years, oncological pediatric surgeons accepted the idea that children could tolerate single, sequential or repeated thoracotomy. The issue of lung volume loss has been faced using nonanatomic parenchymal-sparing resections (wedge or precision resections).
The improvement of knowledge on natural history of many different tumors and the development of systemic treatments led to significant progress in the treatment of non-metastatic tumors.
Ranges of overall survival vary from 20% to 70% for patients with metastatic disease, depending on primary histology.
In this scenario, the indication of surgical treatment of pulmonary metastasis is often evaluated case-by-case, according to histology and prognostic groups. For this reason, the management of these patients is optimal in large experienced centers with a systematic multidisciplinary approach.
Indications
Surgical eligibility criteria, as well as long-term survival rates, differ in various tumors.
Patients are usually accepted in case of local control of primary tumor, in absence of metastases in other organs and when a complete resection of metastases is achievable (3). However, criteria for resection remain undefined in pediatric patients (8-9).
Few general principles were established: number and locations of lung deposits should not contraindicate the operation, the disease-free interval is an important prognostic factor but not an essential criterion (3).
The main factors of selection are the histology and the possibility to achieve a complete resection with an acceptable residual respiratory function, although exceptional decisions are possible for particular cases. In the large and heterogeneous family of pediatric solid tumors, every histology could deserve particular considerations. However, authors considered tumors more frequently treated with a unanimous approach.
Hence, in the group of Pediatric Sarcomas, pulmonary metastasectomy has a well-defined role in soft tissues and osseous tumors, such as osteosarcoma, but it is still controversial in other tumors, like Ewing’s sarcoma and rhabdomyosarcoma.
Osteosarcoma is the typical pediatric histology for which metastasectomy is certainly indicated and long-term results have been clearly reported. Even if it is a rare disease, it is the most common bone’s tumor in pediatric age and adolescence, with an overall incidence of 0.2–3 per 100,000 per year (9). About 15–20% of patients have clinically detectable metastasis at the diagnosis, most commonly in lungs (85–90%) (10,11). Pulmonary metastases have a major impact on the prognosis and metastatic disease has a very poor prognosis with a 5-year overall survival of 20–40% compared to 40–70% of all osteosarcoma patients (9,12). Pulmonary metastasectomy is a common practice for this disease, and many series have been reported in literature in order to identify prognostic factors associated to long-term survival. First of all, in 1971 Martini et al. (7) reported a survival of 27% at 5 years in patients with pulmonary metastasis treated with surgical resection. In the following decades, Beattie and colleagues provided data on the long-term follow-up of this historical cohort. Survival at 10 and 15 years was 27%, and 13.6% after 20 years (13).
In the same years, similar results were obtained by Pastorino et al. who reported the real benefit of salvage surgery on a consecutive series of primary metastatic osteosarcoma in the setting of aggressive and multidisciplinary approach. The systematic salvage surgery combined with an effective chemotherapy demonstrated the survival’s improvement. An aggressive approach resulted to be effective in improving survival, which reached 68% and 58% after 3 and 5 years respectively (14).
Starting from this study, many authors supported with their works the role of pulmonary metastasectomy in prolonging survival, as a part of multidisciplinary approach (9,10,12).
An evidence of micronodular pulmonary diffusion is often considered a surgical exclusion criterium for the difficulty to achieve a complete resection preserving an adequate pulmonary function. Nevertheless, in osteosarcoma the deposit of a calcific matrix allows the palpation of very small lesions, up to a size of 1 mm. Some authors report in literature of 142 resections performed on a single lung (9). An open surgical technique is preferred because the whole lung can be explored, in accordance with data supporting the idea that pre-operative CT scan underestimate tumoral deposits found with palpation (15). For osteosarcoma, authors prefer to perform single or staged thoracotomy (9).
Soft tissues sarcomas of pediatric age, like synovial cell sarcoma, fibrosarcoma, malignant fibrous histiocytoma may metastasize to lung and surgery is normally admitted when this is the only site of dissemination and primary tumor is locally controlled. These rare tumors are often studied in series including heterogeneous histology and their prognostic factors are not clearly defined. Excluding Ewing’s sarcoma (the only radio-sensitive tumor), metastasectomy represents the only chance to obtain complete response to multimodal treatments. The approach is the same for adult soft tissues sarcomas: all nodules detected with palpation are resected; notably these lesions are softer than osteosarcoma’s, then more difficult to be localized (2).
Surgery is more controversial in other pediatric mesenchymal tumors like Ewing’s sarcoma and rhabdomyosarcoma: for these tumors chemotherapy has the main role, while surgery is reserved to specific cases or used for diagnosis.
In Ewing’s sarcoma, there is no clear evidence of surgery benefits on metastatic disease because it is a chemo and radio-sensitive tumor. For this reason, the role of surgery is limited only in residual disease to reach a pulmonary complete response, without any proved impact on survival (16).
Furthermore, the excision of small dubious lesion has a role in diagnosis of benign lesions and preserves patients from the toxicity of useless therapy.
Metastatic rhabdomyosarcoma has a poor outcome but good response to chemotherapy and radiation. For this reason, pulmonary resection plays a role mainly with a diagnostic purpose (17), but is not excluded in presence of isolated and stable metastases with an acceptable disease-free interval.
When surgery is limited to biopsy or to treat a single peripheral nodule, mini-invasive approach [video-assisted thoracic surgery (VATS)] is justified.
Chemotherapy has the main role also in others primary tumors like Wilms, hepatoblastoma, neuroblastoma and adrenocortical carcinoma, where the treatment of a disseminated disease depends overall on the effectiveness of systemic therapy and surgery is reserved to specific cases.
Wilms tumor presents lung metastases in 10% of cases and chemotherapy associated to whole lung irradiation has been demonstrated to produce a good response influencing a good prognosis (18,19). For this reason, lung resection is not indicated in treatment regimen as standard therapy.
However, interstitial pneumonitis occurring as complication of radiant treatment, encouraged some European centers to explore the role of pulmonary metastasectomy (19,20), observing a good overall survival as result of complete surgical remission after initial chemotherapy.
Patients affected by metastatic hepatoblastoma have a lower survival rate in comparison with those without. Despite some trials showed a complete response to chemotherapy, metastasectomy for residual disease is considered essential for long-term survival (21,22).
Neuroblastoma is rarely associated to lung metastases and when they are present are much likely to have other locations. Surgery is indicated with a diagnostic purpose to proceed with a systemic treatment (23).
According to the Memorial Sloan-Kettering Cancer Center (MSKCC) experience, lung metastasectomy is justified even in young patient affected by metastatic adrenocortical carcinoma, even if there are not pediatric series published (3). Adrenocortical carcinoma is rare chemotherapy and radiation-resistant tumor and adult’s experience evidence that this procedure can enhance long-term survival (24,25).
Technical issues/surgical techniques
Patients are subjected to a general anesthesia and intubated in order to obtain an intra-operative single-lung ventilation. The pulmonary complete collapse in operative field allows the whole lung palpation and the occult nodules’ detection. In teenagers, lung collapse is achievable with smaller double-lumen tubes, which usually require the aid of pediatric bronchoscope to be positioned. In younger children, double-lumen tubes are too large and the positioning of a bronchial blocker is preferred. The blocker is positioned under-vision by a pediatric bronchoscope, obtaining a selective bronchial exclusion.
Post-operative pain management can be controlled with an epidural catheter, usually placed after the induction of anesthesia at the end of the operation. If the placement of the catheter is refused or not feasible, intravenous drugs are administered using an elastomeric pump or by a patient-controlled analgesia (PCA) in motivated and collaborative older children and adolescent.
In our experience, an open approach is preferred. The patient is positioned on lateral decubitus, exposing the lateral chest wall by arm abduction on an arch or on a pillow.
A minimally-invasive lateral thoracotomy is performed, with a complete muscle-sparing, in our opinion the best tolerated technique to control pain and obtain a rapid discharge of the patient, even in case of planned staged thoracotomy (26).
Nodules are removed through precision resections using electrocautery or laser methods in order to ensure radical surgery with adequate margins while preserving the surrounding parenchyma and causing a limited volumetric distortion as compared with staplers. With these techniques, it is possible to preserve the surrounding parenchyma and cause a limited volumetric distortion as compared with staplers.
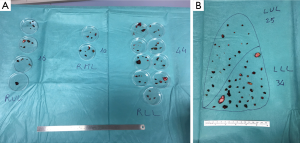
Parenchymal residual defects are closed by single or bidirectional locked suture of 3-0 of polypropylene. When there are several resections (Figure 1A,B), it is impossible to close every hole without impairing postoperative lung function and expansion: in these patients we prefer to close the deeper defects, leaving the more superficial resected areas open (9).
The use of staplers to perform wedge resection should be reserved for patients with few, localized lesions. In children, the kind of stapler depends on the thickness of the pulmonary parenchyma and cartridges. They may vary from 2.5 mm for peripheral nodules to 3.5 mm for more central wedge resections or for closing a central bronchus.
Anatomical resections (segmentectomy, lobectomy or pneumonectomy) should be reserved for selected cases, since major lung resection can only be justified if it leads to a real oncological advantage.
Pleurectomy in presence of pleural deposits is not indicated in most pediatric tumors and when suspicious of pleural disease occurs an intra-operative histologic evaluation is performed, and if positive, the resection should be interrupted.
The aim of surgery is the complete resection of all nodules: a metastasectomy should be planned with this purpose. Partial debulking is admitted only in case of intra-operative evaluation of non -operability after having performed an initial resection.
Thanks to advances in thoracic imaging in the last decade, surgeons should replace the habit of conducting surgical explorations in patients with radiological complete remission, with short-term serial helical-CT scans radiological follow-up.
Repeated thoracotomies are possible: the surgical access and the extension of resection are conditioned from the previous resections and from the location of relapses. There is no theoretical limit to reinterventions: it depends on the natural history of the disease and essentially on the residual respiratory function and not on technical difficulties when being performed by an expert surgeon.
Outcomes
Modern surgical techniques, anaesthesiologic procedures including pain control, introduction peri-operative kinesis-therapy in a controlled and equipped environment allow a reduction of surgical complications and a rapid discharge of children subjected to pulmonary metastasectomy.
Peri-operative mortality is rarely described while surgical complications rate can vary from 0–12% (2,10,27) and include air leak, pneumonia, respiratory insufficiency and superficial wound infection.
With a careful postoperative monitoring, the only frequent issue is the management of chest tubes in presence of reduced residual lung expansion due to the iatrogenic inelasticity of the resected and then sutured parenchyma. When this is the only open issue, the pleural tube can be left in place after the patient is discharged, and then checked and removed when possible in an outpatient setting.
After pulmonary metastasectomy for cancer, Pulmonary function can be impaired in patients in treatment and also in adult survivors (28,29), who often present restrictive syndrome and decreased physical function.
Survival outcomes are various in consideration of different histology.
Surgery continues to be fundamental for metastatic and relapsing osteosarcoma patients and for patients with metastatic soft tissues sarcomas. The complete surgical resection of all sites of disease remains a predictor of survival; repeated metastasectomy can prolong survival or even cure some patients (2,30).
Conclusions
Despite progresses made in treatment of pediatric solid tumors, in children with metastatic disease, outcomes and survival rate cannot be considered optimal. Surgery of lung metastases has a therapeutic and curative role for some histology with acceptable rate of complications or sequelae, but surgeons find their role only in the context of an aggressive multimodality approach. Indications can be discussed case by case and exceptions can be evaluated also in presence of different evidences. Children affected by a metastatic tumor need to be managed by expert multidisciplinary oncological team in referral center with large experience.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heaton TE, Davidoff AM. Surgical treatment of pulmonary metastases in pediatric solid tumors. Semin Pediatr Surg 2016;25:311-7. [Crossref] [PubMed]
- Temeck BK, Wexler LH, Steinberg SM, et al. Metastasectomy for Sarcomatous Pediatric Histologies: Results and Prognostic Factors. Ann Thorac Surg 1995;59:1385-9; discussion 1390. [Crossref] [PubMed]
- Kayton ML. Pulmonary metastasectomy in pediatric patients. Thorac Surg Clin 2006;16:167-83. vi. [Crossref] [PubMed]
- Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913-9. [Crossref] [PubMed]
- Cliffton EE, Pool JL. Treatment of lung metastases in children with combined therapy. Surgery and/or irradiation and chemotherapy. J Thorac Cardiovasc Surg 1967;54:403-21. [PubMed]
- Kilman JW, Kronenberg MW, O'Neill JA Jr, et al. Surgical resection for pulmonary metastases in children. Arch Surg 1969;99:158-65. [Crossref] [PubMed]
- Martini N, Huvos AG, Miké V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg 1971;12:271-80. [Crossref] [PubMed]
- Briccoli A, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol 2010;19:193-9. [Crossref] [PubMed]
- Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther 2016;16:543-56. [Crossref] [PubMed]
- Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006;41:194-9. [Crossref] [PubMed]
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776-90. [Crossref] [PubMed]
- Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005;23:559-68. [Crossref] [PubMed]
- Beattie EJ Jr. Surgical treatment of pulmonary metastases. Cancer 1984;54:2729-31. [Crossref] [PubMed]
- Pastorino U, Gasparini M, Tavecchio L, et al. The contribution of salvage surgery to the management of childhood osteosarcoma. J Clin Oncol 1991;9:1357-62. [Crossref] [PubMed]
- Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg 2006;41:200-6; discussion 200-6. [Crossref] [PubMed]
- Raciborska A, Bilska K, Rychłowska-Pruszyńska M, et al. Management and follow-up of Ewing sarcoma patients with isolated lung metastases. J Pediatr Surg 2016;51:1067-71. [Crossref] [PubMed]
- Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol 2004;22:4787-94. [Crossref] [PubMed]
- de Kraker J, Lemerle J, Voûte PA, et al. Wilm's tumor with pulmonary metastases at diagnosis: the significance of primary chemotherapy. International Society of Pediatric Oncology Nephroblastoma Trial and Study Committee. J Clin Oncol 1990;8:1187-90. [Crossref] [PubMed]
- Green DM, Breslow NE, Ii Y, et al. The role of surgical excision in the management of relapsed Wilms' tumor patients with pulmonary metastases: a report from the National Wilms' Tumor Study. J Pediatr Surg 1991;26:728-33. [Crossref] [PubMed]
- Ehrlich PF, Hamilton TE, Grundy P, et al. The value of surgery in directing therapy for patients with Wilms' tumor with pulmonary disease. A report from the National Wilms' Tumor Study Group (National Wilms' Tumor Study 5). J Pediatr Surg 2006;41:162-7; discussion 162-7. [Crossref] [PubMed]
- Uchiyama M, Iwafuchi M, Naito M, et al. A study of therapy for pediatric hepatoblastoma: prevention and treatment of pulmonary metastasis. Eur J Pediatr Surg 1999;9:142-5. [Crossref] [PubMed]
- Matsunaga T, Sasaki F, Ohira M, et al. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int 2003;19:142-6. [PubMed]
- Kammen BF, Matthay KK, Pacharn P, et al. Pulmonary metastases at diagnosis of neuroblastoma in pediatric patients: CT findings and prognosis. AJR Am J Roentgenol 2001;176:755-9. [Crossref] [PubMed]
- Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 1999;6:719-26. [Crossref] [PubMed]
- Kwauk S, Burt M. Pulmonary metastases from adrenal cortical carcinoma: results of resection. J Surg Oncol 1993;53:243-6. [Crossref] [PubMed]
- Durkovic S, Scanagatta P. Muscle-Sparing Thoracotomy: A Systematic Literature Review and the “AVE” Classification. J Surg Surgical Res 2015;1:35-44. [Crossref]
- Erginel B, Gun Soysal F, Keskin E, et al. Pulmonary metastasectomy in pediatric patients. World J Surg Oncol 2016;14:27. [Crossref] [PubMed]
- Green DM, Zhu L, Wang M, et al. Pulmonary Function after Treatment for Childhood Cancer. A Report from the St. Jude Lifetime Cohort Study (SJLIFE). Ann Am Thorac Soc 2016;13:1575-85. [Crossref] [PubMed]
- Denbo JW, Zhu L, Srivastava D, et al. Long-term pulmonary function after metastasectomy for childhood osteosarcoma: a report from the St Jude lifetime cohort study. J Am Coll Surg 2014;219:265-71. [Crossref] [PubMed]
- Abel RM, Brown J, Moreland B, et al. Pulmonary metastasectomy for pediatric solid tumors. Pediatr Surg Int 2004;20:630-2. [Crossref] [PubMed]


