Epidemiology, microbiology and treatment implications in adult patients hospitalized with pneumonia in different regions of China: a retrospective study
Introduction
Pneumonia is the most common and serious lower respiratory tract infectious disease worldwide, and is a leading cause of mortality, accounting for approximately 3.1 million deaths globally in 2012 (1). The aetiology of bacterial pneumonia varies depending on its origin. Approximately 10 bacterial species have been identified as significant pathogens in community-acquired pneumonia (CAP) in Europe, North America and Australia (2-4). In a 2002 review of 41 European studies, Streptococcus pneumoniae was the most frequent bacterial pathogen in hospitalized CAP patients, followed by Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella species and Haemophilus influenzae (2). Data regarding the bacterial aetiology of hospital-acquired pneumonia (HAP) are limited, and establishing the aetiological agent(s) of HAP can be problematic as it is often not possible to distinguish between colonisation and infection. Gram-negative bacteria are implicated in the majority of HAP cases in Europe and the USA (55–85%), while Gram-positive pathogens (predominantly Staphylococcus aureus) account for 20–30% of cases (3).
A recent review of Asian studies revealed important geographical differences in microbiological aetiology in Asia when compared with Europe and the USA (4). Higher rates of aerobic Gram-negative bacterial involvement in CAP have been reported in Asia in comparison to Europe and the USA (5-7). In addition, S. pneumoniae, the most important CAP pathogen in European studies (2,8), was less commonly isolated from patients in Asia (4). Gram-negative pathogens are predominant causative agents of HAP both in Asia and the West (9,10); however relatively higher incidences of Acinetobacter species, and a lower prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infection have been reported in Asia (9). In addition to the considerable geographical variation in pneumonia aetiology, changes in microbial ecology over time are frequently observed within individual healthcare facilities, and may be influenced by patient populations, ethnicity, seasonal changes and the microbiological techniques used. On-going surveillance and epidemiological studies are therefore necessary to monitor changes in the antimicrobial susceptibility of pathogens over time, and enable the selection of optimal antimicrobial treatment (11). Although pneumonia is considered as a deadliest infectious disease globally, its pathogenicity, treatment patterns and clinical outcomes for hospitalised adult pneumonia patients in China are not well studied nor reported.
Hence, we conducted a retrospective hospital-based study between 2008–2014, involving two major hospitals in southern and northern China (Guangdong Provincial Hospital of Chinese Medicine and Peking University People’s Hospital) to evaluate the epidemiology, microbiological and treatment patterns and clinical outcomes of pneumonia in Chinese population.
Methods
Study subjects and design
This was a retrospective, non-interventional study to assess epidemiology, clinical management and outcomes of adult patients hospitalized with pneumonia using data from four hospitals (Dade Road General Hospital, Fangcun, University City, and Ersha Island) belonging to a single healthcare conglomerate, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China as well as Peking University People’s Hospital, Beijing, China.
Electronic medical records of unique hospitalization cases of patients’ aged ≥18 years with an ICD diagnosis code of pneumonia, interstitial pulmonary disease/other lung infection with a positive bacterial culture, or influenza with no virus identified prior to discharge between 2008 and 2013 at the Guangdong hospitals, and between 2010 and 2014 at the Beijing hospital were reviewed and extracted for analysis. Data for paediatric patients were reported separately. Data from the Guangdong and Beijing study sites were analysed separately to enable qualitative and quantitative comparison.
Ethical considerations
Individual patient data were anonymized and ethical approval was granted by the relevant institutional ethics board committees at each site. This study involved the collection of existing data and records. Informed consent was exempted according to the decision of institutional ethics board committees.
Data collection
Study variables included patient baseline demographics, medical history (including comorbidities and surgical intervention); treatment patterns (antibiotic usage) and clinical outcomes. Demographic information extracted for analysis included age, gender and ethnic origin. Clinical outcomes data extracted for analysis included length of hospital stay, recurrence of infection, intensive care unit (ICU) admission, discharge from hospital and in-hospital mortality. Microbiological data, including bacteria identified, and antibiotic resistance based on microbiological analysis of patient sputum/respiratory secretions and blood samples (collected when possible/as clinically indicated), were also extracted. Bacteria were identified according to Clinical and Laboratory Standards Institute (CLSI) guidelines (12). All bacteria were identified by culture methods, except for Mycoplasma pneumoniae (M. pneumoniae) in Beijing, which was identified using serological methods. Minimum inhibitory concentrations (MICs) were determined by local laboratories using Siemens Microscan Walkaway 96 Plus. Antibiotic susceptibility was interpreted using the current CLSI MIC breakpoints at the time the test was conducted (13-20).
Statistical analysis
All the descriptive analysis was conducted using R3.1.1 (http://www.cran.r-project.org/web/packages/). The mean differences among continuous variables between two groups were tested with the Student’s t-test or the Wilcoxon rank sum test when appropriate. A Fisher’s Exact Test was employed to test the differences in categorical variables between groups. P values of <0.05 were considered statistically significant.
Results
Baseline demographics
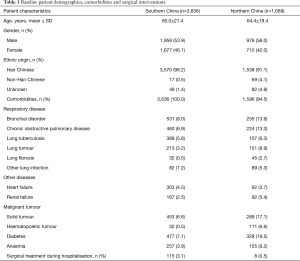
The study population comprised 3,636 and 1,689 unique hospitalization cases diagnosed with pneumonia at admission, of whom 150 (4.1%) and 191 (11.3%) were diagnosed with HAP in Guangdong and Beijing respectively. No information was available for the remaining hospitalization cases regarding diagnosis of either HAP or CAP. Baseline demographics and details regarding comorbidities and surgical interventions are shown in Table 1. The most common comorbidities were bronchial disorder, diabetes, and chronic obstructive pulmonary disease in both populations, with additional solid tumours seen predominantly in Beijing hospitalization cases. According to the comorbidity profiles (see Table 1), patients in Beijing were much sicker than those in Guangdong.
Full table
Microbiological characteristics
Microbiological tests were performed on samples from 1,301 to 1,303 hospitalization cases in southern and northern regions respectively. In patients from Guangdong, a total of 452 samples were culture-positive, the majority of which [399 (88.3%)] were collected from sputum, 37 (8.2%) from blood, and nine (2.0%) from pharyngeal swabs. Less than 2% of culture positive samples were obtained by other techniques, such as bronchoalveolar lavage (BAL) and chest drain. In patients from Beijing, a total of 660 samples were culture-positive, the majority of which [476 (72.1%)] were collected from sputum, 78 (11.8%) from secretions obtained via bronchoscopy, 49 (7.4%) from blood and 49 (7.4%) from pharyngeal swabs. Only eight (1.2%) samples were collected from BAL. In Guangdong and Beijing respectively, a total of 219/686 (31.9%) and 310/945 (32.8%) of the bacterial organisms were isolated from samples collected within 2 days of admission.
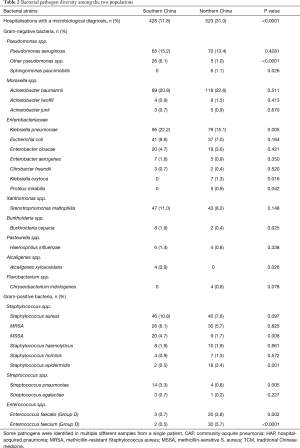
In Guangdong patients, at least one bacterial organism was identified in 428 hospitalization cases (11.8%), of which 318 (74.3%) had a single bacterial organism identified and 110 (25.7%) had >1 bacterial organism identified. The most frequently isolated organisms were Gram-negative bacteria, including Klebsiella pneumoniae [95/428 (22.2%)], Acinetobacter baumannii [89/428 (20.8%)], and Pseudomonas aeruginosa [65/428 (15.2%); Table 2]. Of the Gram-positive bacteria, S. aureus [46/428 (10.8%)] was most common. Fourteen of the 428 (3.3%) hospitalization cases had S. pneumoniae isolated. M. pneumoniae was not detected in any patient.
Full table
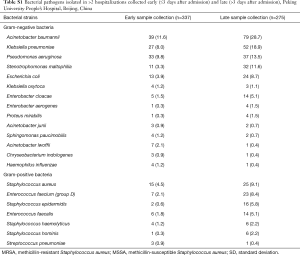
A similar pattern was observed in Beijing patients, where at least one bacterial organism was identified in 523/1,689 (31.0%) hospitalization cases; 128 (24.5%) had >1 bacterial organism identified and the remainder had a single bacterial organism identified. A. baumannii was the most commonly isolated bacteria, identified in 118/523 (22.6%) hospitalization cases, followed by K. pneumoniae [79/523 (15.1%)] and P. aeruginosa [70/523 (13.4%)] (Table 2). The majority of positive bacterial cultures were obtained from samples collected >3 days after admission (Table S1). S. aureus was the most common Gram-positive bacteria accounting for 40/523 (7.6%) of hospitalisations with ≥1 bacterial organism identified; 30 (5.7%) hospitalization cases had MRSA. Only four of the 523 (0.8%) hospitalization cases had S. pneumoniae isolated. Antibodies were identified in 35/861 (4.1%) hospitalization cases whose blood was tested for antibodies against M. pneumoniae. Hospitalizations with microbiological diagnosis were significantly more frequent in Beijing compared to Guangdong (P<0.0001) (Table 2).
Full table
Resistance rates to key antimicrobial agents for S. aureus, Enterobacteriaceae, A. baumannii and P. aeruginosa between 2008–2010 and 2011–2013 are shown in Figure 1 and Figure 2 cultured from Guangdong and Beijing hospitalisation cases respectively.
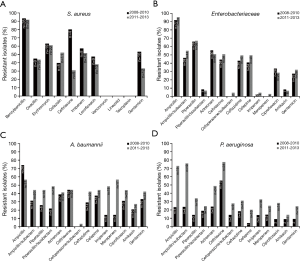
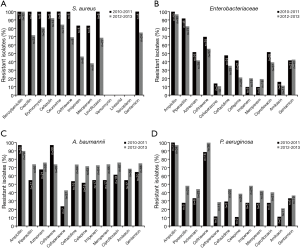
Clinical outcomes and treatment patterns
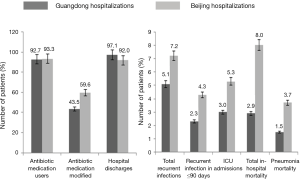
Clinical outcomes of Guangdong and Beijing hospitalization cases are detailed in Figure 3. The mean (standard deviation; SD) length of hospital stay was 12.1 (9.1) days and 20.8 (21.1) days for Guangdong and Beijing patients respectively. The majority of hospitalization cases (97.1%, south; 92%, north) were discharged. Among the Guangdong hospitalization cases, 105/3,636 (2.9%) died; 53 of these deaths (1.5%) were attributable to pneumonia. Recurrence of infection occurred in 172/3,393 (5.1%) patients for whom relevant data were available. Seventy-seven (44.8%) cases of infection recurrence occurred within 90 days. In Beijing hospitalization cases, 135/1,689 (8.0%) died; 63 of these deaths (46.7%) were attributable to pneumonia. Recurrence of infection occurred in 107/1,483 (7.2%) patients for whom relevant data were available. Sixty-four cases (59.8%) of infection recurrence occurred within 90 days. There were no differences in bacteria isolated or clinical outcomes among these patients (data not shown), compared with the overall population.
An institutional guideline was used to determine whether patients were eligible for antibiotic therapy. In both regions, hospitalizations with positive cultures for S. aureus, A. baumannii, P. aeruginosa, K. pneumoniae and E. coli were associated with more frequent modification of initial antibiotic therapy, higher in-hospital mortality, and longer length of stay compared with the overall population (Table S2).

Full table
The majority of hospitalization cases {[3,372/3,636 (92.7%)] and [1,575/1,689 (93.3%)] in Guangdong and Beijing regions respectively} received antibiotic therapy. The choices of antibiotics for initial and overall therapy are presented in Table 3. It was found that the strongest antibiotics vancomycin and cefepime, the fourth generation cephalosporins were only used to treat 9.7% and 8.3% patients in Beijing.
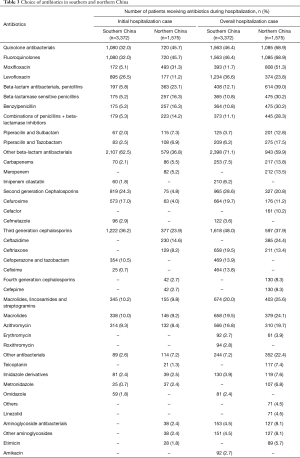
Full table
A total of 1,466 hospitalization cases (43.5%) had their initial treatment modified. Traditional Chinese Medicine (TCM) was used in 3,481/3,636 (95.7%) hospitalization cases in Guangdong; of these, 3,246 (93.2%) received TCM in combination with an antibiotic. Seven cases (0.2%) received TCM without conventional antimycotic, antiviral or antibiotic therapies. Medication information was not available for 22/3,636 (0.6%) hospitalization cases.
In Beijing, initial antibiotic treatment modification occurred in 938/1,575 (59.6%) hospitalization cases who received antibiotic therapy. TCM was not used at the Beijing hospital. In total, 1,589/1,689 (94.1%) patients were treated with conventional antibiotic, antimycotic, or antifungal therapies; the remaining 100 (5.9%) patients were given other medications, including blood substitutes/perfusion solutions, antineoplastic agents, drugs for acid-related disorders, and cardiac therapy.
Discussion
This retrospective, observational study provides detailed information on patient demographics, microbiological characteristics and treatment patterns, and highlights certain similarities and key differences between the epidemiology of pneumonia in patients admitted to hospitals in different regions of China. The average age of patients was 60.0±21.4 and 64.4±18.4 years in Guangdong and Beijing, respectively. Mean length of hospital stay was 12.1 and 20.8 days, and overall mortality was 2.9% and 8.0%, respectively. Similar spectra of bacteria were isolated at both hospitals, with Gram-negative bacteria being predominant. The Gram-negative bacilli that were most frequently isolated included A. baumannii, K. pneumoniae and P. aeruginosa. The high isolation rate of A. baumannii and P. aeruginosa is interesting, given that these are well-known HAP pathogens (21-24), but uncommonly involved in CAP. K. pneumoniae is commonly involved in both CAP and HAP in Asia, Europe and North America, with the highest association with CAP evident in Asia and developing countries (25-27).
S. aureus was the most commonly isolated Gram-positive bacteria in both hospitals. S. aureus is recognized as an important cause of both HAP and CAP (28). The proportion of MRSA among S. aureus isolates was higher in Beijing (75%) as compared with Guangdong (56%). Clinical outcomes were worse in hospitalizations with positive cultures for S. aureus than in the overall population, and were particularly unfavourable for MRSA as compared with methicillin-sensitive S. aureus.
The low frequency of identification of S. pneumoniae in China is notable, given that it is considered to be the most important CAP pathogen in Europe and North America (2). S. pneumoniae is a fastidious organism that may not survive during transportation to the laboratory for analysis (29). At both hospitals, it is standard practice to wait for several samples to be collected before sending specimens for microbiological analysis, potentially reducing the culture viability of fragile bacteria such as S. pneumoniae. In addition, neither hospital performed pneumococcal urinary antigen tests, which may have contributed to the low rate of identification of S. pneumoniae relative to the West, where guidelines recommend routine pneumococcal urinary antigen testing in addition to blood cultures in hospitalized patients with CAP (30). In China, urinary antigen testing is not a regular test and is expensive for patients. Most hospitals rarely perform urinary antigen testing. Pneumococcal vaccination is uncommon in these areas of China, and therefore cannot explain the low frequency of S. pneumoniae isolation.
Antibiotic resistance among Enterobacteriaceae, A. baumannii and P. aeruginosa generally increased between 2008–2010 and 2011–2013. In contrast, antibiotic resistance among S. aureus generally decreased between 2008–2010 and 2011–2013. Several factors suggest that patients’ disease severity was greater in Beijing when compared with Guangdong. Given the similar spectrum of bacteria isolated, this is likely to reflect the fact that Beijing is a tertiary referral facility for patients requiring specialist care, whereas Guangdong is a local hospital with a less severely ill case load, rather than their different geographical locations. Deaths occurred more frequently in Beijing (8%) compared with Guangdong (3%); however, the proportion of deaths due to pneumonia in hospitalization cases with MRSA infection was higher in Guangdong (19%) compared with Beijing (7%), which may again reflect the specialist nature of the Beijing hospital. The average length of stay was considerably longer at Peking University People’s Hospital (approximately 21 days) than at Guangdong Provincial Hospital of TCM (approximately 12 days). Overall, the length of stay across both sites was higher than that reported in a large European epidemiology study (12.6 days) (28). There was a higher frequency of initial antibiotic modification in Beijing (60%) than in Guangdong (43%), which may reflect differences in disease severity, but both were considerably higher than that reported in a similar observational study of hospitalised CAP patients in Europe (29%) (28). However, it is important to note that empiric therapy was only modified if patients later received a microbiological diagnosis and/or the initial treatment was not effective, and in the current study the modifications were mostly de-escalations of antibiotic therapy based on microbiology test results.
This study has several strengths, particularly the inclusion of data from two large healthcare conglomerates in two regions of China, which helped in portraying the pneumonia story of the entire country. The study focuses on the adult population affected with pneumonia, and provides valuable information on antibiotic usage, bacterial aetiology, antimicrobial resistance patterns and clinical outcomes, which adds to the very scarce data already available. Importantly, given the observed differences in China as compared with Europe/North America, treatment recommendations in Western pneumonia management guidelines (30,31) may need modification for use in China and other Asian countries to avoid adverse consequences, such as lack of antimicrobial efficacy, increased morbidity and mortality, and the development of antimicrobial resistance.
Limitations of this study include the retrospective design which includes uncertainty regarding the case definitions; inconsistencies in recording patient data and microbiological testing methods; lack of HAP or CAP diagnosis; and the lack of urinary antigen testing for atypical pathogens such as Mycoplasma pneumoniae. Specimen collection following antibiotic use, or delays in specimen transport, might have affected the levels of isolation of specific bacteria.
Conclusions
Of all pathogens identified, Staphylococcus aureus infection (particularly with methicillin-resistant S. aureus) was associated with poor clinical outcomes in both regions; however antibiotic resistance among S. aureus generally decreased during the study data collection periods. This study also shows that disease severity was greater in Beijing as compared with Guangdong and this may be associated with higher microbiological diagnosis rate and higher frequency of initial antibiotic modification among Beijing populations. This comprehensive data on the microbiology, aetiology, treatment pattern and clinical outcomes among the two regions of China may help to develop appropriate treatment and improve the detection, prevention and control of pneumonia in Chinese adults.
Acknowledgements
We thank Liz Bolton and Mark Waterlow of Prime Medica Ltd., Knutsford, Cheshire, UK; and Dr. Amit Bhat of Indegene Pvt. Ltd., Bangalore, India for providing medical writing support. The authors would like to acknowledge Tong Zhu of Yidu Cloud Technology Co., Ltd., who contributed to the extraction, integration and linking of data from electronic medical records. This study was funded by AstraZeneca.
Footnote
Conflicts of Interest: Jesus Gonzalez and Judith Hackett were employees of AstraZeneca at the time of the study. The remaining authors declare that they have no conflicts of interest.
Ethical Statement: The study was approved by institutional ethics committee/ethics board of Guangdong Provincial Hospital of Chinese Medicine (No. AF/01-05.0/13.0) and Peking University People’s Hospital (No. 2013PHB011-01).
References
- World Health Organization. The 10 leading causes of death in the world, 2000 and 2012. Fact sheet n°310. World Health Organization 2014.
- Woodhead M. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur Respir J Suppl 2002;36:20s-7s. [Crossref] [PubMed]
- Lynch JP 3rd. Hospital-acquired pneumonia: risk factors, microbiology, and treatment. Chest 2001;119:373S-84S. [Crossref] [PubMed]
- Peto L, Nadjm B, Horby P, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg 2014;108:326-37. [Crossref] [PubMed]
- Brown JS. Geography and the aetiology of community-acquired pneumonia. Respirology 2009;14:1068-71. [Crossref] [PubMed]
- Liam CK, Pang YK, Poosparajah S, et al. Community-acquired pneumonia: an Asia Pacific perspective. Respirology 2007;12:162-4. [Crossref] [PubMed]
- Song JH, Thamlikitkul V, Hsueh PR. Clinical and economic burden of community-acquired pneumonia amongst adults in the Asia-Pacific region. Int J Antimicrob Agents 2011;38:108-17. [PubMed]
- Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-54. [Crossref] [PubMed]
- Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control 2008;36:S93-100. [Crossref] [PubMed]
- Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005;41:848-54. [Crossref] [PubMed]
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138-80. [Crossref] [PubMed]
- Clinical and Laboratory Standard Institute. Abbreviated identification of bacterial and yeast; approved guideline. In: Document m35-a2, 2nd edition. Wayne, PA: Clinical and Laboratory Standards Institute, 2015.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 18th informational supplement. Clsi document m100-s18. Wayne, PA: Clinical and Laboratory Standards Institute, 2008.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. Clsi document m100-s19. Wayne, PA: Clinical and Laboratory Standards Institute, 2009.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 20th informational supplement. Clsi document m100-s20. Wayne, PA: Clinical and Laboratory Standards Institute, 2010.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. Clsi document m100-s21. Wayne, PA: Clinical and Laboratory Standards Institute, 2011.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. Clsi document m100-s22. Wayne, PA: Clinical and Laboratory Standards Institute, 2012.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. Clsi document m100-s23. Wayne, PA: Clinical and Laboratory Standards Institute, 2013.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 24th informational supplement. Clsi document m100-s24. Wayne, PA: Clinical and Laboratory Standards Institute, 2014.
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard - 9th edition. Clsi document m07-a9. Wayne, PA: Clinical and Laboratory Standards Institute, 2012.
- Gupta D, Agarwal R, Aggarwal AN, et al. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: Joint ICS/NCCP(I) recommendations. Lung India 2012;29:S27-S62. [Crossref] [PubMed]
- Kaul R, Burt JA, Cork L, et al. Investigation of a multiyear multiple critical care unit outbreak due to relatively drug-sensitive acinetobacter baumannii: Risk factors and attributable mortality. J Infect Dis 1996;174:1279-87. [Crossref] [PubMed]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538-82. [Crossref] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Bansal S, Kashyap S, Pal LS, et al. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci 2004;46:17-22. [PubMed]
- Huang HH, Zhang YY, Xiu QY, et al. Community-acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patients. Eur J Clin Microbiol Infect Dis 2006;25:369-74. [Crossref] [PubMed]
- Reechaipichitkul W, Lulitanond V, Tantiwong P, et al. Etiologies and treatment outcomes in patients hospitalized with community-acquired pneumonia (CAP) at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health 2005;36:156-61. [PubMed]
- Blasi F, Garau J, Medina J, et al. Current management of patients hospitalized with community-acquired pneumonia across Europe: outcomes from REACH. Respir Res 2013;14:44. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Identification and Characterization of Streptococcus pneumoniae. In: Laboratory methods for the diagnosis of meningitis caused by neisseria meningitidis, streptococcus pneumoniae, and haemophilus influenzae. 2011. Available online: http://www.cdc.gov/meningitis/lab-manual/chpt08-id-characterization-streppneumo.html
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]