The challenge of co-existent moderate aortic stenosis and left ventricular systolic impairment
Introduction
Aortic stenosis (AS) is the commonest cause of valvular heart disease in the western world with an estimated prevalence of up to 7% in patients aged 65 years or over (1). Characterised predominantly by progressive valve fibrosis and calcification, leading to restriction of the aortic valve cusps, the severity of AS has traditionally been defined by haemodynamic parameters assessed either invasively, or more commonly using echocardiography. In the setting of clinically significant AS, reactive hypertrophy of the left ventricle (LV) occurs in response to the associated LV pressure overload, maintaining wall stress and cardiac performance for many years if not decades. Ultimately, however, this process decompensates and patients transit from hypertrophy to symptomatic heart failure with poor clinical outcomes unless the valve is replaced.
Severe AS is commonly defined using 3 echocardiographic parameters, a peak aortic trans-valvular velocity >4 m/s, a mean gradient >40 mmHg and an aortic valve area <1.0 cm2 (2). However, in reality, the physiological and myocardial impact of a given degree of valve stenosis is likely determined by multiple other factors including the systemic blood pressure, gender, genetic influences and coexistent cardiac pathology. This is reflected in the clinical course of AS, as many patients do not immediately develop symptoms on transitioning to severe AS and the time course and peak velocity at which symptoms and LV decompensation develop in individual patients is highly variable.
According to our existing understanding, patients with moderate AS should represent a stable asymptomatic group with a good prognosis about whom clinicians need not worry about until progression to severe disease. However, a recent study by van Gils et al. has suggested that, at least in patients with impaired LV systolic function, moderate AS may not be as benign a condition as first thought (3).
Published in the Journal of the American College of Cardiology, van Gils et al. performed an observational cohort study of patients across four tertiary centres and three countries to evaluate clinical outcomes in patients with moderate AS and impaired LV systolic function [ejection fraction (EF) <50%]. The patient population was representative of general clinical practice (average age of 73 years, 75% male) with extensive medical comorbidities consistent with their elderly composition. One in ten patients were found not to have significant AS at all, but reduced valve opening and a falsely low aortic valve area at rest due to their low flow status.
After 4 years of follow-up, an adverse event [death, admission with heart failure admission or aortic valve replacement (AVR)] had occurred in 61% of patients with moderate AS and LV impairment. Approximately 1 in 4 patients underwent AVR (mostly surgical valve replacement). Even after exclusion of AVR as an outcome, rates of all-cause mortality (36%) and hospitalisation for HF remained high (27%). The strongest independent predictors of adverse outcome were New York Heart Association functional class III or IV (HR 2.86) and peak aortic velocity (HR 2.24 per 1 m/s increment).
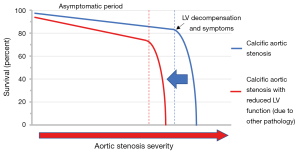
Based on their findings, van Gils and colleagues conclude that moderate AS associated with LV impairment may not be a benign condition (Figure 1). Indeed the event rates observed in this study are broadly similar to contemporary heart failure populations without AS despite the comparatively modest reductions in EF (4,5). However, this patient group is highly heterogeneous making it difficult to determine whether the adverse prognosis relates to the AS and associated afterload, the impaired LV function, or the condition underlying the development of LV dysfunction. Without this understanding, it may be difficult to determine the optimal management strategy. We suggest that when assessing patients with moderate AS and LV impairment it is critical to address the following 3 key clinical questions as outlined below.
Is moderate AS really severe AS?
Determining the severity of AS severity can be extremely challenging, particularly when LV function is impaired. An underestimation of AS severity could potentially account for some of the excess hazard observed by van Gils and colleagues. The severity of AS is defined by three haemodynamic parameters assessed on echocardiography: peak velocity, mean gradient and aortic valve area. Discordance amongst these measures (e.g., a low valve area but low peak velocity) is common (6) particularly in the context of low flow states (e.g., impaired systolic function, a small LV cavity or with co-existent mitral regurgitation). This is compounded by inherent inaccuracies in the measurements used to assess severity; for example, acceptable sonographic windows are required for optimal assessment of peak velocity and this may be challenging in patients with obesity or chronic lung disease. In addition, calculation of aortic valve area using the continuity equation requires accurate measurement of left ventricular outflow tract (LVOT) diameter, which, can be difficult with poor images and in the presence of LVOT calcification. Moreover, the equation assumes a circular diameter whereas the LVOT is elliptical and any errors in diameter measurements are compounded when squared to provide an area (7-9).
Most measures also ignore the influence of patient size. A given aortic valve area may represent mild stenosis in a large male, but severe stenosis in a small female and indexing measures to body surface area may improve accuracy. In the current study, the authors re-evaluated their population using indexed thresholds and found that up to a third of patients subsequently met the indexed criteria for severe AS (<0.6 cm/m2). Thus, a number of potential pitfalls exist that may lead to an underestimation of the severity of AS. In this context, the relatively high incidence of clinical events observed by van Gils et al. is perhaps not as surprising as initially thought.
These problems are inherent in all AS studies and alternative approaches may be required to arbitrate disease severity, particularly when echocardiographic measures are discordant. One emergent technique of potential use is the computed tomography (CT) aortic valve calcium score, which is flow independent, has gender specific thresholds and is a strong predictor of adverse events (10).
What is the mechanism of LV dysfunction?
Traditional thinking teaches us that moderate AS does not cause LV dysfunction which, if present, must reflect an alternative aetiology. Whilst certain patients can demonstrate evidence of LV decompensation with only moderate AS (11), effort needs to be made to identify other causes of LV decompensation. This is especially important given the multiple co-morbidities that frequently co-exist with AS including hypertension and ischemic heart disease. In older patient cohorts, other conditions such as cardiac amyloidosis are increasingly recognised as a cause of LV impairment (12).
Distinguishing the aetiology of LV impairment will likely play a key role in determining which patients, if any, may gain benefit from AVR in the setting of moderate AS and LV impairment. For example, the finding of Q waves on the ECG or regional wall thinning and akinesis in a coronary distribution on echocardiography may suggest infarcted myocardium due to coronary artery disease. Although the majority of patients had coronary disease in this study and 48% were defined as having ischemic cardiomyopathy, it is not clear how this was characterised. Other imaging technologies such as cardiac magnetic resonance (CMR) may help, with the late gadolinium enhancement technique reliably able to differentiate between scar due to myocardial infarction, co-existent cardiomyopathies and amyloidosis (13). Moreover mid-wall fibrosis on CMR is increasingly being recognised as a specific marker of LV decompensation in AS (11,14) which may guide the timing of AVR or transcatheter aortic valve implantation (TAVI). This hypothesis is currently being examined in the EVOLVED trial (NCT03094143).
Will AVR or TAVI improve patient outcomes?
Clinical outcomes following AVR or TAVI in patients with moderate AS and LV impairment are likely to reflect a balance between the potential hemodynamic benefits following relief of outflow tract obstruction, and the risks of any corrective procedure. Where AS is felt to be the predominant cause of the LV decompensation, and the latter is potentially reversible, then the decision in favour of intervention may be justified. Similarly, in AS patients with ischemic cardiomyopathies and myocardial viability then coronary artery bypass grafting (CABG) and concomitant AVR is likely to be beneficial.
What about patients with irreversible myocardial damage due to an alternative pathology? Traditionally these patients have not been referred for surgery given that they may have a higher procedural risk and less to gain from an aggressive interventional approach. However, it has been argued that the reduction in afterload associated with valve replacement might allow for improved myocardial performance and improved outcomes even in this patient group. With the development of less invasive options for AVR including transfemoral TAVI, the potential for harm is reduced, potentially shifting the balance in favour of valve intervention.
Patients with moderate AS and LV dysfunction are therefore a heterogeneous and controversial group. van Gils and colleagues suggest as a group that they may represent a population who would generally gain benefit from valve intervention. In support of this, a retrospective observational study from the Duke Echocardiographic Laboratory Database, followed 1,090 patients with moderate AS and LV dysfunction over 5 years with 26% patients ultimately undergoing AVR. Those patients who received AVR had a reduced risk of death (HR 0.57) compared with no intervention that persisted after multivariable adjustment (15). Even with complex statistical modelling, however, observational non-randomized studies remain confounded by clear biases in the selection of patients for AVR. Moreover, we lack reliable outcome data in each of the different subgroups outlined above that might help us to better stratify those patients who would and would not benefit from intervention.
Until we have robust randomised data examining these strategies in detail, severe AS will remain the primary indication for AVR and TAVI. As intriguing as the data presented by van Gils and colleagues are, we believe there is insufficient evidence to entertain a one-size-fits-all approach of recommending AVR in all patients with moderate AS and LV dysfunction and would advocate a patient stratified approach after clarifying the severity of AS, the mechanism of LV dysfunction, and the likelihood of LV recovery post valve intervention.
Conclusions
The study by van Gils and colleagues highlights the importance of identifying LV dysfunction in patients with moderate AS. It seems plausible that within this heterogeneous population, some patients may benefit from early valve intervention while others will not. A patient stratified approach, with careful assessment of the severity of AS, the aetiology of LV dysfunction, and the potential for recovery, remains central to interventional decision making. Upcoming randomised controlled trials (such as TAVR UNLOAD) may provide further evidence to help guide treatment strategies in this complex group of patients. Until then, the indications for AVR and TAVI will remain predominantly in the domain of patients with severe AS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012;59:235-43. [Crossref] [PubMed]
- van Gils L, Clavel MA, Vollema EM, et al. Prognostic Implications of Moderate Aortic Stenosis in Patients With Left Ventricular Systolic Dysfunction. J Am Coll Cardiol 2017;69:2383-92. [Crossref] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, Enalapril, or Aliskiren and Enalapril in Heart Failure. N Engl J Med 2016;374:1521-32. [Crossref] [PubMed]
- Clavel MA, Messika-Zeitoun D, Pibarot P, et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329-38. [Crossref] [PubMed]
- Messika-Zeitoun D, Serfaty JM, Brochet E, et al. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol 2010;55:186-94. [Crossref] [PubMed]
- De Vecchi C, Caudron J, Dubourg B, et al. Effect of the ellipsoid shape of the left ventricular outflow tract on the echocardiographic assessment of aortic valve area in aortic stenosis. J Cardiovasc Comput Tomogr 2014;8:52-7. [Crossref] [PubMed]
- Chin CW, Khaw HJ, Luo E, et al. Echocardiography underestimates stroke volume and aortic valve area: implications for patients with small-area low-gradient aortic stenosis. Can J Cardiol 2014;30:1064-72. [Crossref] [PubMed]
- Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202-13. [Crossref] [PubMed]
- Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271-9. [Crossref] [PubMed]
- Nietlispach F, Webb JG, Ye J, Cheung A, et al. Pathology of transcatheter valve therapy. JACC Cardiovasc Interv 2012;5:582-90. [Crossref] [PubMed]
- Kelle S, Roes SD, Klein C, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 2009;54:1770-7. [Crossref] [PubMed]
- Chin CW, Everett RJ, Kwiecinski J, et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc Imaging 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Samad Z, Vora AN, Dunning A, et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J 2016;37:2276-86. [Crossref] [PubMed]


