Incidence and risk factors of iatrogenic pneumothorax after thoracentesis in emergency department settings
Introduction
Thoracentesis is a commonly performed procedure for diagnostic or therapeutic purposes in up to 173,000 cases in the United States annually (1-3). Though it is generally considered safe and has a low risk during the procedure, complications have been reported (4). Iatrogenic pneumothorax is the most common complication following thoracentesis, leading to increased morbidity, mortality, and health care costs due to increased length of hospital stay (5,6). It requires the insertion of a chest tube for up to 4 days in up to half of the cases, further increasing hospital stays, resulting in an additional increase in cost of approximately 18,000 dollars (5,7,8).
Previous studies have reported the incidence of thoracentesis-related pneumothorax as 4–30% without the use of ultrasound and 1.3–6.7% with the use of ultrasound (9-11). A systematic review and meta-analysis of 24 studies, including a total of 6,605 cases that received thoracentesis, reported an overall 6.0% incidence of pneumothorax (5,12). However, there are a few studies of the incidence of pneumothorax in patients in emergency department (ED) settings, where thoracentesis is commonly used.
Clinical practice that incorporates system changes focused on high-quality training, referrals to experienced practitioners and team-centered procedure care have been shown to reduce complications following thoracentesis (13,14). A recent study of 9,320 thoracentesis in 4,618 patients has reported a 0.61% incidence of thoracentesis-related pneumothorax (15). Accordingly, routine chest radiography may not be required following thoracentesis in asymptomatic patients in cases where the primary purpose of post-procedural radiography is to identify thoracentesis-related pneumothorax. However, the low incidence of pneumothorax observed in this previous study may not apply to general populations as all thoracentesis were performed by expert practitioners.
In the present study, we determine the incidence of pneumothorax following thoracentesis in an ED setting not by procedure team and evaluate the association of specific demographics, clinical, and procedure factors with thoracentesis-related pneumothorax.
Methods
Study design and population
This retrospective study was conducted in the academic ED of a tertiary care, university-affiliated hospital in Seoul, Korea, which cares for approximately 110,000 patients per year. The electronic medical records of all consecutive adult (≥18 years) patients with pleural effusion who underwent thoracentesis in the ED of our hospital between January 2009 and December 2014 were examined. The patients who underwent thoracentesis regardless of purpose (diagnosis or treatment) and drain effusion amount were included. Patients with evidence of pneumothorax prior to thoracentesis on initial plain chest radiography were excluded.
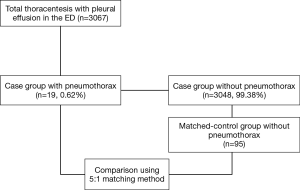
The incidence of pneumothorax following thoracentesis was investigated. The diagnosis of pneumothorax was based on plain chest radiography performed routinely within 1 hour of the procedure in our ED to identify post-procedural complication. A total of 19 patients who developed pneumothorax following thoracentesis were identified. Each case was matched to five controls, which did not develop pneumothorax following thoracentesis, according to the following criteria: same age, same sex, and same attendance year. In this manner, 95 controls were matched to 19 patients, resulting in a total study population of 114.
Due to the retrospective nature of the present study, our institutional review board approved the review of patient data before its commencement and waived the requirement for informed consent (approval #2016-1352).
Data collection
The clinical and demographic characteristics of all included patients, including their age, sex, comorbidities, height, weight, body mass index (BMI), laboratory findings, amount of drained pleural effusion, site of thoracentesis (bilateral or unilateral), the use of invasive or noninvasive ventilation, frequency of needle pass, guidance of ultrasound (real-time or site marking), purpose of thoracentesis, pleural effusion loculation, and operator specialty were retrieved from the electronic hospital records.
Pleural effusion was diagnosed by plain chest radiography or computed tomography (CT), and the decision to perform thoracentesis was at the discretion of the treating physician. The decision to use ultrasonography was made according to the opinion of the treating physician. BMI was classified as underweight (<18.50), normal (18.50–24.99), overweight (25.00–29.99), and obese (≥30.00) according to the criteria set by World Health Organization (16,17). Diagnostic thoracentesis was referred to as thoracentesis for diagnosis when manual aspiration was used, and the amount of fluid drained was less than 60 cc. Therapeutic thoracentesis was for the purpose of treatment with a natural drain using an intravenous connector line. A small amount of pleural effusion was defined when there was difficulty in localizing by physical examination and diagnosis was conducted only by chest CT or ultrasound. Loculated effusion was defined as the situation where a fluid that was in a nondependent location exhibited a convex border toward the lung or a mass effect on the adjacent lung (18). Dry tap was defined as no effusion being sampled in at least one try of thoracentesis. No more than 1,500 cc thoracentesis was performed in a single procedure at our ED. Routine plain chest radiography was conducted regardless of patient symptoms, for detecting post-thoracentesis complications.
Statistical analysis
Data are presented as the median with interquartile ranges for continuous variables and as absolute or relative frequencies for categorical variables. Comparison between patients who developed pneumothorax and those who did not was performed using 1:5 case-control matching. The Mann-Whitney U test was used to compare continuous variables. The chi-square tests or Fisher’s exact test were used to compare categorical variables. Factors found to be significantly associated (P≤0.20) with complications in univariate analysis were included in the multivariate analysis. The results of stepwise logistic regression analysis for pneumothorax following thoracentesis were presented as ORs and 95% CIs. P values of ≤0.05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
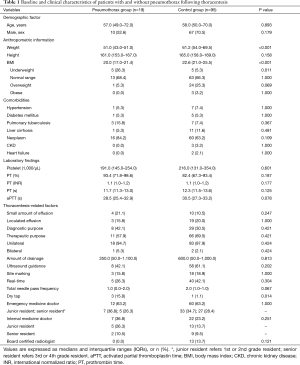
During the present study period, 3,067 thoracentesis for 2,556 patients with pleural effusion were performed in our ED. Of these, iatrogenic pneumothorax following thoracentesis occurred in 19 patients, with an overall incidence of 0.62% (Figure 1). The annual incidence of pneumothorax during the study period was not significantly different (P=0.942). The median age of patients with pneumothorax was 57.0 (49.0–72.0) years, of which 52.6% were male. We conducted 1:5 case-controls matching to identify factors associated with the occurrence of iatrogenic pneumothorax following thoracentesis. In the univariate analysis, weight and BMI were found to be significantly lower in patients who developed pneumothorax (P<0.001 for both; Table 1). No significant differences in the prevalence of comorbidities or laboratory findings were observed between the two groups. Regarding procedure-related factors, a dry tap following thoracentesis was more prevalent in the pneumothorax group (P=0.013). Ultrasound guidance (real-time, site marking both) and specialty of operator did not significantly differ between the two groups.
Full table
Length of hospital stay was longer in the pneumothorax group; however, there difference did not reach statistical significance (Table 2). Chest tube insertion (36.8%) was performed for the management of pneumothorax in the pneumothorax group. No pneumothorax-related operations were required. In-hospital mortality was significantly higher in the pneumothorax group than that in the control group (P=0.014). However, no patient in our study population expired due to pneumothorax occurrence. Among the three patients in the pneumothorax group, the causes of death were as follows: respiratory failure due to aggravation of pleural effusion, progression of lung cancer, and pneumonia. Pneumonia was the cause of death for one patient in the control group. The intensive care unit admission rate did not significantly differ between the two groups.

Full table
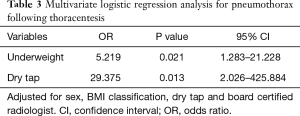
Sex, BMI classification, dry tap, activated partial thromboplastin time, board certified radiologist and total needle pass frequency were identified as significant variables in the univariate analysis (P≤0.20). Sex, BMI classification, board certified radiologist and dry tap were included in multivariate logistic regression analysis to identify independent variables that can predict the occurrence of pneumothorax after thoracentesis. To avoid overfitting problems, we did not include activated partial thromboplastin time and total needle passage frequency. Activated partial thromboplastin time and total needle passage frequency were not included because of lack of clinical relevance and multicollinearity, respectively. Being underweight was found to be independently associated with the occurrence of pneumothorax [OR, 5.219 (95% CI, 1.283–21.228); P=0.021] (Table 3). Dry tap after thoracentesis was also found to be significantly associated with the occurrence of pneumothorax [OR, 29.375 (95% CI, 2.026–425.884); P=0.013]. Board certified radiologist was not a significant factor in multivariate logistic regression analysis (P=0.999).
Full table
Discussion
The overall incidence of iatrogenic pneumothorax following thoracentesis in the ED was 0.62% in the present study. We found that factors associated with the occurrence of pneumothorax following thoracentesis were being underweight and a dry tap. Iatrogenic pneumothorax was required following 36.9% of chest tube insertions and showed higher mortality than that in patients who did not experience pneumothorax following thoracentesis.
To the best of our knowledge, the present study is the first to evaluate the incidence and associated factors of iatrogenic pneumothorax following thoracentesis in ED settings, where thoracentesis is commonly used. Despite previous studies reporting the incidence of pneumothorax as high as 19%, recent studies have found pneumothorax to be uncommon following thoracentesis, with reported incidences ranging from 0% to 3% (6,9,19,20). A recent meta-analysis reported a summarized pneumothorax rate of 6.0%, which is comparatively higher than our result (5). However, overestimation of the pneumothorax rate may have occurred due to heterogeneity across primary studies. Furthermore, studies published after 2000, including more than 200 cases of thoracentesis, have reported significantly lower pneumothorax rates than earlier and smaller studies. A large prospective study performed by dedicated proceduralists reported a pneumothorax rate of 0.6% (15). Though all thoracentesis were performed by a team with hyper-expert hands, the overall incidence of pneumothorax was similar to our result as 63.2% of the thoracentesis in our study were also performed by emergency physicians who frequently perform thoracentesis. Despite the absence of a significant association between operator specialty and the occurrence of pneumothorax, the pneumothorax group had a higher proportion of thoracentesis performed by internal physicians than the control group (36.8% vs. 23.3%). These results indicate that pneumothorax is rare following thoracentesis performed by experienced clinicians. Given the rare incidence of iatrogenic pneumothorax after thoracentesis, routine chest radiography might not be necessary if thoracentesis is performed by an experienced physician.
Underweight patients were found to be five times more likely to experience pneumothorax in the present study, corroborating the results of a previous study (15). This result may be attributed to the fact that underweight patients have a shorter distance between the chest wall and visceral pleura, thereby resulting in an increased risk of underestimating the depth of needle passage or chest wall thickness. Another possible explanation is that their reduced tolerance for invasive procedures due to poor nutritional or overall health status. A dry tap is likely to increase the risk of pneumothorax as it is an indicator of incorrect needle positioning or difficulty of the procedure. However, due to the broad CI and small number of cases (three cases in the pneumothorax group), further studies evaluating dry tap as a risk factor for pneumothorax are required to clarify this issue. Many previous studies have reported the utility of ultrasound guidance in reducing the risk of iatrogenic pneumothorax (6,21-24). However, we did not observe an association between the use of ultrasound guidance and pneumothorax. Although Wilcox et al. reported that ultrasound guidance is not statistically associated with a decreased risk of pneumothorax, they suggested that the benefit of ultrasound differs depending on the amount of effusion or presence of loculation (25). In the present study, analysis including only loculated effusion demonstrated a lower pneumothorax rate with ultrasound guidance, although this difference did not reach statistical significance (33.3% vs. 63.2%). Thus, we believe that ultrasound guidance should be considered when small or loculated effusions are suspected. Furthermore, it seems that it is not appropriate to underestimate the importance of ultrasound guidance for a small number of events in our study.
The major limitations of the present study were the retrospective study design and small case size, which may have reduced the statistical significance of the observed results. Hence, even though we have used the matched design, our results should be interpreted with caution. As no standard protocol was used to determine the indication for thoracentesis, some patients may not have received thoracentesis despite having indications. In contrast, difficult cases may have received thoracentesis, thereby confounding results. Lastly, because a relatively high proportion of patients with neoplasm were included in the pneumothorax group (84.2%), the possibility of secondary pneumothorax with cancer invasion on pleural without direct injury with needle should be considered.
The findings of the present study show that the incidence of iatrogenic pneumothorax following thoracentesis is very low in EDs without hyper-expert team. Clinicians should be aware of the risk of pneumothorax in patients who are underweight and have a dry tap during the procedure and consider the use of plain chest radiography to detect the presence of pneumothorax in such cases. However, given the small case size of the present study, further prospective studies are warranted to clarify these results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Due to the retrospective nature of the present study, our institutional review board approved the review of patient data before its commencement and waived the requirement for informed consent (approval #2016-1352).
References
- Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13 1998.1-119. [PubMed]
- Light RW. Pleural effusions. Med Clin North Am 2011;95:1055-70. [Crossref] [PubMed]
- Feller-Kopman D. Ultrasound-guided thoracentesis. Chest 2006;129:1709-14. [Crossref] [PubMed]
- Diagnostic thoracentesis and pleural biopsy in pleural effusions. Health and Public Policy Committee, American College of Physicians. Ann Intern Med 1985;103:799-802. [Crossref] [PubMed]
- Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010;170:332-9. [Crossref] [PubMed]
- Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest 2013;143:532-8. [Crossref] [PubMed]
- Sassoon CS, Light RW, O'Hara VS, et al. Iatrogenic pneumothorax: etiology and morbidity. Results of a Department of Veterans Affairs Cooperative Study. Respiration 1992;59:215-20. [Crossref] [PubMed]
- Despars JA, Sassoon CS, Light RW. Significance of iatrogenic pneumothoraces. Chest 1994;105:1147-50. [Crossref] [PubMed]
- Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 1990;150:873-7. [Crossref] [PubMed]
- Seneff MG, Corwin RW, Gold LH, et al. Complications associated with thoracocentesis. Chest 1986;90:97-100. [Crossref] [PubMed]
- Gervais DA, Petersein A, Lee MJ, et al. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997;204:503-6. [Crossref] [PubMed]
- Cantey EP, Walter JM, Corbridge T, et al. Complications of thoracentesis: incidence, risk factors, and strategies for prevention. Curr Opin Pulm Med 2016;22:378-85. [Crossref] [PubMed]
- Duncan DR, Morgenthaler TI, Ryu JH, et al. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest 2009;135:1315-20. [Crossref] [PubMed]
- Hooper CE, Lee YC, Maskell NA. Setting up a specialist pleural disease service. Respirology 2010;15:1028-36. [Crossref] [PubMed]
- Ault MJ, Rosen BT, Scher J, et al. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-32. [Crossref] [PubMed]
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [Crossref] [PubMed]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1-253. [PubMed]
- Moulton JS, Benkert RE, Weisiger KH, et al. Treatment of complicated pleural fluid collections with image-guided drainage and intracavitary urokinase. Chest 1995;108:1252-9. [Crossref] [PubMed]
- Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol 2014;12:139. [Crossref] [PubMed]
- Perazzo A, Gatto P, Barlascini C, et al. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol 2014;40:6-12. [Crossref] [PubMed]
- Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003;123:436-41. [Crossref] [PubMed]
- Jones PW, Moyers JP, Rogers JT, et al. Ultrasound-guided thoracentesis: is it a safer method? Chest 2003;123:418-23. [Crossref] [PubMed]
- Raptopoulos V, Davis LM, Lee G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol 1991;156:917-20. [Crossref] [PubMed]
- Barnes TW, Morgenthaler TI, Olson EJ, et al. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005;33:442-6. [Crossref] [PubMed]
- Wilcox ME, Chong CA, Stanbrook MB, et al. Does this patient have an exudative pleural effusion? The Rational Clinical Examination systematic review. JAMA 2014;311:2422-31. [Crossref] [PubMed]