CT angiography for diagnosis and subcategorization of unroofed coronary sinus syndrome
Introduction
Unroofed coronary sinus (CS) is partial or complete absence of the roof of CS (1-4), leading to a communication between CS and left atrium (LA). Unroofed coronary sinus syndrome (URCS) is the rarest type of atrial septal defect (ASD) (1). It comprises less than 1% of all types of ASD (5). URCS has also been described as “occult” left-to-right shunt given the difficulties with echocardiography diagnosis (6,7). Through this defect, a left-to-right or right-to-left shunt occurs (8-12). The simple form of this anomaly should be suspected in patients with an unknown cardiac murmur, left-to-right interatrial shunt, right-sided heart enlargement, pulmonary hypertension (PH), unexplained arterial oxygen desaturation, or cerebral complication, such as brain abscess or cerebral emboli or infarction (1,10,11,13,14).
Before the era of echocardiography, precise diagnosis of this anomaly was only possible during surgical procedure or autopsy. Nevertheless, findings in autopsy and open-heart surgery are unable to reflect the in vivo anatomy and real-time hemodynamics. Precise diagnosis with echocardiography can be difficult in adults owing to the limited acoustic window and contrast resolution (6,7). Due to its superior spatial resolution, CT angiography (CTA) has been outstanding in depicting the anatomy of cardiac veins and signs of congenital heart disease (CHD) (15,16). In the current study, we retrospectively reviewed the imaging findings in 46 patients with URCS to evaluate the effectiveness of CTA for diagnosis and subcategorization of URCS, and to decide if this new subcategorization scheme was relevant to diagnosis and surgical decision-making.
Methods
Demographic and clinical profiles
Between November 2005 and August 2015, 46 patients were diagnosed with URCS by cardiac CTA in Fu Wai Hospital (Beijing, China).
There were 32 adults and 14 children, with a mean age of 31.8±20.5 years (range, 0.4–67 years). Eighteen patients complained of chest discomfort with dyspnea (New York Heart Association functional class I–II). The remaining 28 patients presented with exertional dyspnea or palpitation (New York Heart Association functional class III–IV), and 12 of them were found to have cardiac murmur after birth.
Cardiac CTA and 2D transthoracic echocardiography (TTE) with Doppler imaging were performed in all patients, and transesophageal echocardiography (TEE) in two patients.
Cardiac CTA technique
All pediatric patients underwent preoperative examination by cardiac ECG-gated CTA using dual-source scanner. Iodixanol injection (320 mgI/mL, Visipaque, GE Healthcare, Chicago, IL, USA) was applied using a single-head power injector (Stellant Dual Flow, Medrad, Pittsburgh, PA, USA). The contrast media was injected through the antecubital vein. The volume was adjusted according to the body weight:1.5–2.0 mL/kg. Depending on body weight, iodixanol was injected at a flow rate of 0.8–1.2, 1.2–1.8, 1.8–3.0, 3.0–5.0 mL/s for children aged 0–3, 3–6, 6–12, and more than 12 years, respectively. Bolus-tracking was used in a region-of-interest (ROI) at descending aorta. When attenuation threshold of ROI was more than 80 Hounsfield unit (HU), auto-delayed 6 s were triggered for the automatic scanning. A radiologist and a pediatrician monitored vital signs including ECG and blood oxygen saturation during the examination.
All adult patients underwent preoperative examination by cardiac ECG-gated CTA using either a 64-slice or a 40-row detector dual-source scanner. A double-head power injector (Stellant, Medrad) was used to inject contrast media through a 20-G trocar in an antecubital vein. The volume was adjusted at 1.0–1.2 mL/kg. The maximum amount was 2 mL/kg or less for any patient. Based on body weight, iohexol 350 mgI/mL (Omnipaque 350, GE Healthcare) or iopromide 370 mgI/mL (Ultravist 370, Bayer-Schering Pharma, Berlin, Germany) was injected at a flow rate of 4.0–5.0 mL/s. Bolus-tracking was used in a ROI at ascending aorta. When attenuation threshold of ROI was more than 100 HU, auto-delayed 6 s were triggered for the automatic scanning. One radiologist monitored vital signs including ECG during the examination.
For single source 64-slice CT, helical scanning with 0.16 to 0.22 pitch, 400 mm table feed/rotation, 64 detectors, 0.625 mm individual detector width, and 350 ms gantry rotation time were used. For dual-source CT, axial scanning with 40 detectors, 0.625 mm individual detector width, and 270 ms gantry rotation time were used. In children, tube voltage and tube current were set as follows: 80 kv, 100 mA. In adults, tube voltage was set at 100 kv for routine use, only when the body mass index (BMI) greater than 30 at 120 kv; ECG-modulated tube current ranged from 200–550 mA (tube current was 550 mA during 35–80% R-R interval when diagnostic image quality was required, and remained at 200 mA during the other phases of the R-R interval); the field of view was 200–260 mm.
Postprocessing procedures
Data set was reconstructed, and off-line postprocessing of CTA image was performed on dedicated workstations (ADW version 4.3 or 4.6, GE Healthcare). The thickness of the reconstructed image was 0.625 mm. Two investigators who were experienced in image post-processing and blinded to patient information analyzed all the images.
According to the dedicated protocol in ADW workstation, the center of volume data was put at the point of defect of CS to reformat multi-planner reconstruction (MPR) view with certain spatial angle. When adjustment was required, the user could place new point on the MPR which were used as control point to generate the best MPR view of the defect. The heart isolation protocol in the ADW workstation was used to reformat volume rendering (VR) views.
CTA imaging analysis for subcategorization of URCS
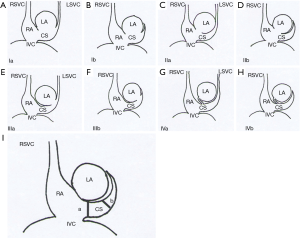
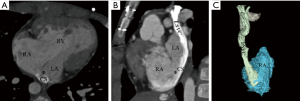
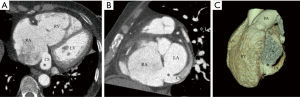
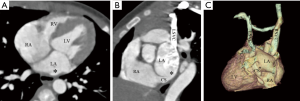
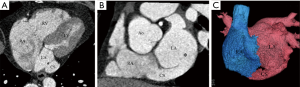
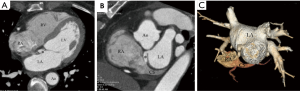
We classified URCS into four types based on the classification system of Kirklin and Barratt-Boyes (17) and that of Xie and associates (18). According to the defect size, URCS can be divided into complete or partial types (Table 1). In complete defect URCS (Figure 1A,B), the CS does not exist, because the common wall between it and the LA is absent. Partial defect URCS is divided into proximal type and distal type. Proximal type is defined as defect location in continuity with the CS ostium (Figure 1C,D). Distal type is defined as defect location in the midportion of roof of CS (Figure 1E,F). Type IV is a variant type with an anomalous vessel connecting CS and LA (Figure 1G,H). Then URCS is classified into the above four types, and each type consists of two subtypes according to the presence of left superior vena cava (LSVC) (Figure 1).
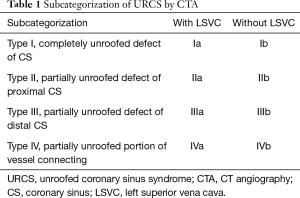
Full table
Calculation of the mean CS diameter indexed to body surface area (CS index)
In this cohort, the diameter of the CS, the location and size of the defect, and its distance from the CS ostium were measured and analyzed (Figure 1). In type I we measured the distance between the inferior edge of the atrial septum and the distal end of the CS defect. In type II we measured the distance between the inferior edge of the atrial septum and the proximal end of the CS defect. In type III we measured the distance between proximal end and distal end of the CS defect, and there was a normal division wall between the proximal CS and LA as shown.
CS index was defined as the mean CS transverse diameter indexed to surface area, i.e. CS diameter/body surface area (mm/m2). We measured the CS transverse diameter at a point of 1 cm to the orifice of the sinus on reformatted short-axis MPR views of cardiac CTA images (Figure 1I) in all types. There is no wall of the left atrium in type I, so the point of 1 cm located at the imaginary straight line between the inferior edge of the atrial septum and the distal end of the CS defect.
In one patient with complete atrioventricular canal and common atrium, measurement was not possible. Therefore, data were available in only 45 patients.
Statistical analysis
All images were analyzed by two cardiovascular radiologists until they reached a consensus. Statistical analyses were performed using SPSS for Windows 17.0 (SPSS Inc., Chicago, IL, USA). All statistical analyses were two sided and any P value of less than 0.05 was considered statistically significant.
Results
Comparison of CTA and echocardiography in the diagnosis of URCS
In this cohort, URCS was detected with CTA in 46 patients, and 19 of these 46 patients had typical signs of PH. The main pulmonary artery diameter (mPAD) and mPAD/ascending aortic diameter (AAD) ratio are useful parameters for predicting PH on CTA (19).
Only 45.6% (21/46) of patients with URCS was detected by echocardiography (Table 2), including types Ia and Ib in 5 and 6, types IIa and IIb in 1 and 5, types IIIa and IIIb in 1 and 3, respectively. The sensitivity of echocardiography in detecting URCS was significantly lower compared with cardiac CTA (P<0.05).

Full table
Anatomical features of URCS in CTA
Isolated URCS
In this cohort, isolated URCS was detected by CTA in 16 patients. Three of them had previously undergone surgery to treat other congenital cardiac anomalies (ASD in 1, tetralogy of Fallot in 2) and developed symptoms 15 to 30 years later. One in 16 had an associated ostium stenosis of the CS, which showed a diameter of only 3.9 mm and dense contrast entering the RA through the stenosed CS. One patient (type IVb) was found to have a slim tube communication between the CS and LA via an anomalous vessel. According to literature, this variant of URCS is rare up to date (20-22).
URCS with concomitant cardiac deformities
In this cohort, 30 URCS patients with other concomitant congenital heart malformations were detected. Simple CHD was diagnosed in 21 patients, including ASD in 15 patients, VSD in 2, cor triatriatum in 3 and coarctation in 1, respectively. Complex CHD were diagnosed in 9 patients, including partial anomalous pulmonary vein connection (PAPVC) in 4, interrupted aortic arch in 1, partial and total endocardial cushion defect in 1 and 3, respectively.
Anatomic variations of LSVC or LIV
In this series, LSVC was present in 28.3% (13/46) of patients. In 8 of those patients (61.5%, 8/13), the LSVC terminated in the LA; in 9 patients (69.2%, 9/13), the left innominate vein (LIV) was absent in which LSVC formed as the confluence of the left subclavian and left internal jugular vein and drained into the CS.
Subcategorization and CS index in this cohort
According to our new classification system, the URCS was type Ia in 7 patients (Figure 2), type Ib in 16 (Figure 3), type IIa in 3 (Figure 4), type IIb in 7 (Figure 5), type IIIa in 3 (Figure 6), type IIIb in 9 (Figure 7), and type IVb in 1 (Figure 8).
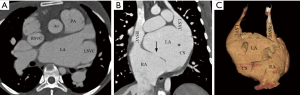
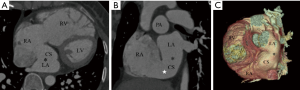
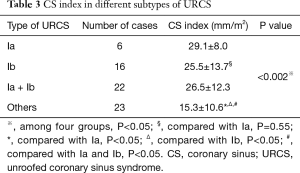
The size of CS defect measured by CTA ranged from 3–65.9 mm. In patients with type Ia and Ib URCS, CS index were 29.1±8.0 and 25.5±13.7 mm/m2, there was no statistical difference between them (P=0.55) (Table 3). In patients with types I, CS index was significantly greater than that of other types (26.5±12.3 versus 15.3±10.6 mm/m2; P<0.05) (Table 3), and greater than the values of hearts with normal anatomy in literature (7.0±2.0 mm/m2; P<0.05) (20,23). In patients with an associated LSVC, CS index was 22.6±9.7 mm/m2, which was similar to patients without LSVC (20.0±13.8 mm/m2; P=0.54).
Full table
Clinical management
Thirty patients in this series underwent surgical repair or transcatheter intervention. Operative reports were available in 22 with description of the management of URCS (Table 4). The surgical repair in patients with types I, II and IIIa differs significantly from patients with types IIIb and IV (P<0.05, Table 4). In the remaining 8 patients, 3 were detected by CTA as URCS with CHD who developed recurrent symptoms 15–30 years and they refused reoperation for URCS this time. The 4 other patients underwent surgical repair of ASD (2, URCS type Ib) and PAPVC (2, URCS type Ib, IIIa, respectively), but no information was available regarding the management of URCS in their operative reports. Another patient was diagnosed with single ventricle, complete atrioventricular canal, pulmonary stenosis and URCS. He underwent a bi-directional Glenn shunt, leaving the URCS and other defects unrepaired.

Full table
Sixteen patients did not have surgical repair. In 6 patients, the malformation was irreparable due to fixed severe PH, with types Ia and Ib in 2 and 4 respectively. The remaining 10 patients were asymptomatic and were regularly followed up, including types IIb in 2, types IIIa and IIIb in 1 and 6 patients respectively and types IVb in 1.
Discussion
In patients with URCS, clinical manifestations vary with the defect size and the degree of left-to-right shunt. However, URCS is very difficult to diagnose based on clinical signs and symptoms alone. TTE is the most widely used imaging modality for suspected URCS, but is limited by its ability to visualize cardiac vessels and the posterior cardiac structures, such as the CS and pulmonary veins, including the URCS. In this cohort, URCS was detected by echocardiography only in 21 patients. Of the remaining 25 patients, it was not possible to distinguish URCS from ASD in 6 cases and detect a left-to-right shunt from the LA into a dilated CS in 19 cases with echocardiography (Table 2).
Easier diagnosis of URCS by CTA
CTA yields better anatomic information in terms of image resolution and reconstruction capability. Axial image is the basic image for diagnosis of URCS. Short axis MPR image based on axial image cardiac construction is called the “CS view”. Cardiac MPR long-axis view is also useful in the diagnosis of URCS because it is perpendicular to the long axis of the CS defect. Direct visualization with a volume-rendered virtual angiography or navigation CTA technique is helpful in delineating the CS defect anatomically. The 3D VR view adds in visualizing the space relation between right coronary artery and dilated CS. The endocardial view is very important for surgical decision-making because of its similarity to the surgeon’s view and its ability to delineate the relation between CS defect and mitral valve.
Easier detection of associated abnormalities by CTA
With CTA scan, it is easier to detect the anatomical variations of LSVC and LIV which are often associated with URCS. In the series of Quaegebeur et al. (24), 75% of patients with URCS had an LSVC. In our series, LSVC was present in 28.3% (13/46) of patients, which is significantly lower than that in the literature. LSVC usually drains into the RA via a CS. Rarely, LSVC connects to the LA. In 61.5% (8/13) of our patients, the LSVC terminated in the LA between the left pulmonary veins, posteriorly, and at the base of the left atrial appendage, anteriorly. In other reports (24,25), LIV was absent in 80–90% of URCS patients with LSVC. In our cohort, the LIV was absent in only 69.2% (9/13) of URCS patients with LSVC, and 19.6% (9/46) of all patients. In our cohort, the incidence of URCS patients with LSVC and LIV is lower than that of other literature reports (24,25), which may be related to ethnic differences.
It should be noted that if LSVC and LIV exist simultaneously, inadvertent LSVC ligation may result in brain and upper limb edema or petechiae (1). So, it is important to diagnose both deformities with CTA scan before surgical repair.
Hemodynamics of URCS detected by CTA
CTA scan can indirectly reflect the hemodynamic changes of URCS. The hemodynamics may be influenced by the following factors in patients with URCS: (I) LSVC; (II) pathology of CS ostium, such as dilation, stenosis or occlusion; (III) atrioventricular communication; (IV) anomalies obstructing systemic blood flow to the RA. To a certain extent, the size of CS also reflects the changes in hemodynamics. URCS should be highly suspected in patient with an enlarged CS without LSVC. If a patient with URCS develops symptoms is considered for surgical repair, further hemodynamic evaluation is warranted to quantify the degree of intracardiac shunt either using Doppler analysis with TTE or TEE, phase-contrast imaging with cardiac MRI, or right cardiac catheterization.
Clinical management
In our subcategorization system of URCS, patients with type I, II and IIIa URCS usually have more severe clinical symptoms, and the surgical repair in patients with type I, II and IIIa differ significantly from patients with type IIIb and IV. Therefore, surgical repair is more likely in patients with types I, II and IIIa at an earlier age from the perspective of radiology and imagology.
In 3 patients with CHD who had surgical repair 15–30 years ago and developed recurrent symptoms, the diagnosis of URCS was confirmed by CTA. The clinical symptoms may be associated with URCS which was missed by previous surgery. So, occult left-to-right shunt of URCS may persist many years and the heart continue to be overloaded, until relevant clinical symptoms occur again. Another 4 surgical patients who had been preoperatively diagnosed with URCS by CTA, no information was available regarding the management of URCS in their previous operative reports. It’s worth noting that URCS may be missed by imaging or even during surgical repair due to its hidden location (26-28). The chances of leaving URCS untreated during surgical operation was possibly even higher, which occurred in 7 out of 30 patients in this series. In clinical settings, when CTA detects a URCS which was not surgically repaired during previous operation, close follow-up of such patients is warranted.
There are several limitations of this study. One concern pertains to the hemodynamic characteristics of URCS, which could not be delineated by CTA directly. Due to the retrospective nature of this study, further observation in a larger series is warranted to evaluate the role of CTA in the diagnosis of URCS and the applicability of our subcategorization system.
Conclusions
According to our subcategorization system of URCS based on CTA findings, CS index was significantly greater in patients with types I than that of other types. Patients with types I, II and IIIa often have more severe clinical symptoms and require surgical repair earlier from the perspective of radiology and imagology. Given that URCS may be neglected in preoperative diagnosis or initial repair, CTA has the advantage of detecting URCS more easily. This subcategorization system is helpful for delineating the anatomical features and hemodynamic characteristics of URCS and helpful in surgical-decision making.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Fu Wai Hospital, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 2017-920). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Ootaki Y, Yamaguchi M, Yoshimura N, et al. Unroofed coronary sinus syndrome: diagnosis, classification, and surgical treatment. J Thorac Cardiovasc Surg 2003;126:1655-6. [Crossref] [PubMed]
- Huang XS. Images in cardiovascular medicine. Partially unroofed coronary sinus. Circulation 2007;116:e373. [Crossref] [PubMed]
- Low SC, Oliveira GR, Maki JH. Magnetic resonance imaging of unroofed coronary sinus. Heart 2009;95:720. [Crossref] [PubMed]
- Choe YH, Kim YM, Han BK, et al. MR imaging in the morphologic diagnosis of congenital heart disease. Radiographics 1997;17:403-22. [Crossref] [PubMed]
- Ngee T, Lim MC, De Larrazabal C, et al. Unroofed coronary sinus defect. J Comput Assist Tomogr 2011;35:246-7. [Crossref] [PubMed]
- Hahm JK, Park YW, Lee JK, et al. Magnetic resonance imaging of unroofed coronary sinus: three cases. Pediatr Cardiol 2000;21:382-7. [Crossref] [PubMed]
- Brancaccio G, Miraldi F, Ventriglia F, et al. Multidetector-row helical computed tomography imaging of unroofed coronary sinus. Int J Cardiol 2003;91:251-3. [Crossref] [PubMed]
- Chin AJ, Murphy JD. Identification of coronary sinus septal defect (unroofed coronary sinus) by color Doppler echocardiography. Am Heart J 1992;124:1655-7. [Crossref] [PubMed]
- Cochrane AD, Marath A, Mee RB. Can a dilated coronary sinus produce left ventricular inflow obstruction? An unrecognized entity. Ann Thorac Surg 1994;58:1114-6. [Crossref] [PubMed]
- Raghib G, Ruttenberg HD, Anderson RC, et al. Termination of Left Superior Vena Cava in Left Atrium, Atrial Septal Defect, and Absence of Coronary Sinus; a Developmental Complex. Circulation 1965;31:906-18. [Crossref] [PubMed]
- Yilmaz AT, Arslan M, Demirkilic U, et al. Partially unroofed coronary sinus syndrome with persistent left superior vena cava, absent right superior vena cava and right-sided pericardial defect. Eur J Cardiothorac Surg 1996;10:1027-9. [Crossref] [PubMed]
- Troost E, Gewillig M, Budts W. Percutaneous closure of a persistent left superior vena cava connected to the left atrium. Int J Cardiol 2006;106:365-6. [Crossref] [PubMed]
- Cannavale G, Higgins CB, Ordovas KG. Unroofing the diagnosis. Int J Cardiovasc Imaging 2010;26:841-2. [Crossref] [PubMed]
- Oyama N, Ooka T, Sasaki T, et al. Volume-rendering and endocardial views of partially unroofed coronary sinus with 64-slice multidetector CT. J Cardiovasc Comput Tomogr 2009;3:346-7. [Crossref] [PubMed]
- Thangaroopan M, Truong QA, Kalra MK, et al. Images in cardiovascular medicine. Rare case of an unroofed coronary sinus: diagnosis by multidetector computed tomography. Circulation 2009;119:e518-20. [Crossref] [PubMed]
- Shah SS, Teague SD, Lu JC, et al. Imaging of the coronary sinus: normal anatomy and congenital abnormalities. Radiographics 2012;32:991-1008. [Crossref] [PubMed]
- Kirklin JW, Barratt-Boyes BG. Cardiac surgery. Morphology, diagnostic criteria, natural history, techniques, results and indications. New York: Wiley, 1986.
- Xie MX, Yang YL, Cheng TO, et al. Coronary sinus septal defect (unroofed coronary sinus): echocardiographic diagnosis and surgical treatment. Int J Cardiol 2013;168:1258-63. [Crossref] [PubMed]
- Mahammedi A, Oshmyansky A, Hassoun PM, et al. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging 2013;28:96-103. [Crossref] [PubMed]
- Kim H, Choe YH, Park SW, et al. Partially unroofed coronary sinus: MDCT and MRI findings. AJR Am J Roentgenol 2010;195:W331-6. [Crossref] [PubMed]
- Justaniah A, McKee B, Silver J, et al. Coronary sinus to left atrium communication. J Radiol Case Rep 2013;7:16-20. [Crossref] [PubMed]
- Scheller V, Mazur W, Kong J, et al. Coronary sinus to left atrial communication. Case Rep Med 2009;2009:790715.
- Klimek-Piotrowska W, Koziej M, Holda MK, et al. The Thebesian valve height/coronary sinus ostium diameter ratio (H/D-Ratio) as a new indicator for specifying the morphological shape of the valve itself in multisliced computed tomography. Int J Cardiol 2015;201:595-600. [Crossref] [PubMed]
- Quaegebeur J, Kirklin JW, Pacifico AD, et al. Surgical experience with unroofed coronary sinus. Ann Thorac Surg 1979;27:418-25. [Crossref] [PubMed]
- Rastelli GC, Ongley PA, Kirklin JW. Surgical Correction of Common Atrium with Anomalously Connected Persistent Left Superior Vena Cava: Report of Case. Mayo Clin Proc 1965;40:528-32. [PubMed]
- Perez Matos AJ, Planken RN, Bouma BJ, et al. Unroofed coronary sinus newly diagnosed in adult patients after corrected congenital heart disease. Neth Heart J 2014;22:240-5. [PubMed]
- Peighambari M, Esmaeilzadeh M, Alizadehasl A, et al. Partially unroofed coronary sinus, persistent left superior vena cava and cortriatriatum: a rare combination of interruption in normal embryogenesis. Res Cardiovasc Med 2014;3:e15383. [PubMed]
- Yarrabolu TR, Doshi UH, Douglas WI, et al. Unusual presentation of unroofed coronary sinus with cyanosis after ventricular septal defect closure. World J Pediatr Congenit Heart Surg 2015;6:83-5. [Crossref] [PubMed]


