The current status of antiplatelet therapy in patients undergoing transcatheter aortic valve implantation
Transcatheter aortic valve implantation (TAVI) is a minimally invasive and life-saving treatment in patients with aortic valve stenosis. As a consequence of increased operator experience and the refinement of valve technology, the target population of TAVI has rapidly expanded from inoperable patients to individuals with an intermediate risk score (1-3). This evolution of TAVI in the last decade has resulted in an improvement of better quality of life for the majority of the patients (4). Nevertheless, thromboembolic events and bleeding complications associated with TAVI remain an important complication associated with a high morbidity and mortality. In the recent PARTNER trial studying TAVI in intermediate risk patients, the incidence of myocardial infarction at 30 days was 1% and the incidence of neurological events 6%, including 3% disabling stroke (2). These rates were similar in patients undergoing surgical aortic valve replacement. This is in strong contrast with the early days of TAVI. In the pivotal PARTNER-B trial, stroke rates at 1 year follow-up were fivefold higher in patients undergoing TAVI compared to patients treated conservatively (1). As a result of these concerning outcomes, guidelines recommend anti-coagulation both during the procedure as well as an antithrombotic regimen after TAVI by means of several months of dual antiplatelet therapy (DAPT) (5). Nevertheless, in the absence of randomized data, the current guidelines are based upon empirical information rather than evidence based data. In the current editorial we discuss the latest evidence on antiplatelet therapy in patients undergoing TAVI and provide an outlook on future trials.
The present expert-opinion based guidelines of DAPT are not in accordance with combined evidence of current available studies. A pooled analysis of individual patient data by Hassell et al. included 435 patients from 4 studies (2 randomized controlled trials and 2 matched cohorts) (6). It was concluded that addition of clopidogrel versus treatment with aspirin alone did not reduce stroke or mortality rates during the first month after TAVI. Additionally, there was a trend towards less life-threatening and major bleedings in patients treated with single antiplatelet therapy (SAPT, OR 0.56, 95% CI: 0.28–1.11, P=0.09).
Continuing the quest for the optimal antiplatelet therapy after TAVI, the ARTE trial (aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve implantation) is an open-label pilot-trial in patients undergoing TAVI with a balloon expandable valve, evaluating the risk/benefit ration of DAPT vs. SAPT (7). We congratulate the authors of the ARTE trial for conducting a very relevant and well-presented investigator-initiated trial. This multicentre trial by Rodés-Cabau et al. included 222 patients between 2012 and 2017 and was prematurely stopped at 74% of the intended population, due to slow enrolment. Patients were randomized to receive SAPT (N=111, aspirin or acetylsalicylic acid 80–100 mg/day for at least 6 months) or DAPT (N=111, aspirin or acetylsalicylic acid 80–100 mg/day for at least 6 months plus clopidogrel 75 mg for 3 months). Surprisingly, outcomes at 30 days showed a trend towards higher event rates in the DAPT arm compared to treatment with SAPT regarding both myocardial infarction [OR 4.13 (0.45–37.60), P=0.18] as well as neurological events [OR 3.11 (0.32–30.43), P=0.31]. Nevertheless, these outcomes should be interpreted with caution given the combination of relatively low event rates (3 patients with neurological events in the DAPT group vs. 1 in the SAPT group) and the modest sample size of the current study. This stroke rate (1.8% of the study population) is considerably lower than the stroke rates of the earlier discussed large scale prospective trials, despite comparable patient characteristics. A more compelling result is the threefold higher incidence of major or life-threatening bleeding events [OR 3.22 (1.01–10.34), P=0.04] in in the DAPT group. No new thromboembolic events and major bleedings happened between 30 days and 3 months in either of the groups. These major bleedings are of concern since they are identified as a strong independent predictor of mortality in the medium-term follow-up (adjusted hazard ratio: 3.91, 95% CI: 2.67–5.71, P<0.001) (8).
Rodés-Cabau et al. hypothesize that the absent benefit of several months of DAPT may be explained by the time course of the development of these thromboembolic events. Indeed, 2 out of 3 strokes in the first year after TAVI occur within the first 30 days. Approximately a quarter of all strokes at 30 days is observed in the first 24 hours (1,9) and more than half between days 1 to 5 (1). Accordingly, it is likely, that the increased thromboembolic risk for the largest part is more or less directly associated to the TAVI procedure itself. This is in accordance with the first PARTNER trial, where stroke rates between 30 days and 1 year were comparable in patients undergoing TAVI and patients treated conservatively (1). After the peri-procedural period, the risk of stroke seems predominantly influenced by the preexisting predisposition of a TAVI population composed of octogenarians with a typically concomitant adverse cardiovascular risk profile. In contrast, in a subgroup, the risk of stroke may be enhanced by new onset atrial fibrillation after TAVI. However in an observational study on this subgroup of patients, concomitant antiplatelet therapy use did not reduce the incidence of thromboembolic events after TAVI, while it did significantly increase the risk on major bleedings.
To better understand this early peak of stroke and in order to examine potential therapeutic possibilities we have to look in more detail to the underlying pathophysiology of thromboembolic events (Figure 1). During TAVI, the use of large-sized delivery systems and catheters and, balloon valvuloplasty, positioning and implantation of the new valve and post-dilation manipulates the calcified native valves and the aortic wall. Consequently, potential dislodgement and embolization of aortic debris and crushed calcified native valves can take place. Moreover, the thrombophilic state induced by the devices used during TAVI may stimulate thrombus formation through platelet aggregation and subsequent activation of the coagulation pathway. This is confirmed by studies that quantified the etiology of embolization during TAVI by extracting debris from cerebral protection filters (10-12). Frequently found types of debris consisted of arterial wall tissue (52–94%), native valve tissue (20–60%), calcifications (50–73%) and surprisingly foreign material, detached from the percutaneous devices (10–36%). Interestingly, histopathologic debris was found in nearly all cerebral protective devices. The bulk of the debris extracted from the filters contained acute thrombi, implying inappropriate antithrombotic measures, only a few organizing thrombi were captured (6–33%), indicating it was already attached to the native valve or aortic wall prior to the TAVI.
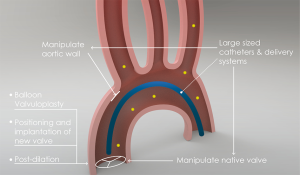
It can be hypothesized that in order to prevent this peak of serious thromboembolic complications, the focus should be on the peri-procedural phase rather than the months after the procedure. Therapeutic options currently explored are the use of cerebral protection devices and antithrombotic measures peri-procedural. Diverse cerebral protection devices have been developed to reduce cerebrovascular events after TAVI. These devices work through deflection or filtering of the embolism and are in different stages of testing. So far, the use of two devices (Sentinel Cerebral Protection System, Claret Medical Inc. and TriGuard, Keystone Heart) has proven to be feasible during TAVI (10,13). Improved versions of the current devices are under development and studies examining efficacy are ongoing. Also addressing the peri-procedural period, the BRAVO-3 trial randomized patients to TAVI with either bivalirudin or unfractionated heparin (control), cardiovascular events, bleeding rates and cerebral embolism on MRI scans were comparable in both groups (14,15).
So what will the (near) future bring us? Under the hypothesis that thrombin is a key-player in the pathophysiology of thromboembolic events the GALILEO study (clinicaltrials.gov, NCT02556203) is currently randomizing 1520 patients to receive either rivaroxaban and acetylsalicylic acid or standard therapy with DAPT. In addition, the POPular TAVI trial is a large randomized trial (clinicaltrials.gov, NCT02247128), continuing on the path of monotherapy. This study is divided into two cohorts including more than 1,000 patients, in cohort A, patients without anticoagulation are randomized to receive SAPT or DAPT. In cohort B, patients on oral anticoagulation are randomized to receive additional clopidogrel or no additional antiplatelet therapy. If the POPular TAVI trial will confirm SAPT is non-inferior to DAPT, and associated with lower rates of bleedings, the initiation of (national) prospective randomized clinical registries could be the next step in the determination of personalized optimal antiplatelet therapy after TAVI.
In conclusion, the ARTE-trial provides important hypothesis-generating information on the significantly higher bleeding rates associated with DAPT after TAVI. However, it does not (yet) provide a reliable conclusion regarding the potential advantages reducing myocardial infarction and stroke, due to the limited sample size in combination with relatively low event rates. Nevertheless, the current study is probably the first step, followed by large-scale randomized studies and a change in daily clinical practice as the end result.
Acknowledgements
We would like to acknowledge and thank Michael R. Jenkins for creating the illustration for the current editorial.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Baron SJ, Arnold SV, Wang K, et al. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol 2017;2:837-45. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200-54. [Crossref] [PubMed]
- Hassell ME, Hildick-Smith D, Durand E, et al. Antiplatelet therapy following transcatheter aortic valve implantation. Heart 2015;101:1118-25. [Crossref] [PubMed]
- Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv 2017;10:1357-65. [Crossref] [PubMed]
- Genereux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol 2014;64:2605-15. [Crossref] [PubMed]
- Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [Crossref] [PubMed]
- Kapadia SR, Kodali S, Makkar R, et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2017;69:367-77. [Crossref] [PubMed]
- Schmidt T, Schluter M, Alessandrini H, et al. Histology of debris captured by a cerebral protection system during transcatheter valve-in-valve implantation. Heart 2016;102:1573-80. [Crossref] [PubMed]
- Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013;127:2194-201. [Crossref] [PubMed]
- Lansky AJ, Schofer J, Tchetche D, et al. A prospective randomized evaluation of the TriGuard HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J 2015;36:2070-8. [Crossref] [PubMed]
- Dangas GD, Lefevre T, Kupatt C, et al. Bivalirudin Versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement: The Randomized BRAVO-3 Trial. J Am Coll Cardiol 2015;66:2860-8. [Crossref] [PubMed]
- Van Belle E, Hengstenberg C, Lefevre T, et al. Cerebral Embolism During Transcatheter Aortic Valve Replacement: The BRAVO-3 MRI Study. J Am Coll Cardiol 2016;68:589-99. [Crossref] [PubMed]


