Thermal ablation for the treatment of primary and secondary pulmonary malignancies
In the review article entitled “The Role of Percutaneous Image-Guided Thermal Ablation for the Treatment of Pulmonary Malignancies” (1) recently published in the American Journal of Roentgenology (AJR) by Mouli and colleagues, the authors reviewed thermal ablation techniques [radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation] for the treatment of pulmonary malignancies [non-small cell lung cancer (NSCLC) and metastasis] with respect to treatment mechanism, local efficacy, imaging modalities used for guidance, treatment response evaluation, and clinical outcomes. The authors also reviewed the comparative studies of thermal ablations with surgery and stereotactic beam radiotherapy (SBRT).
Ablation is a good candidate for treating pulmonary malignancies because lungs have heat and electrical insulating characteristics, which enable larger volumes of tissue to be ablated using thermal energy than is possible for other body tissues (2). Because thermal energy delivery is limited by the heat-sink effect of adjacent blood vessels and airways, the presence of vessels or bronchi greater than 3 mm in diameter within the ablation zone are predictors of incomplete local treatment (3). Ablation zones must exceed tumor dimensions to obtain adequate margins, and in practice, sufficient RFA-induced ground-glass opacity (GGO) indicates complete ablation (4). MWA permit larger ablation zones than RFA because delivers energy simultaneous using multiple probes, and thus, provides larger tumor ablation volumes and faster ablation times. Cryoablation uses compressed argon gas to generate subzero temperatures, but unlike heat-based ablation, cryoablation does not create GGO; instead, it creates iceballs.
CT is the preferred imaging modality for guidance during thermal ablation. It provides excellent contrast between tumors and normal lung parenchyma, and multi-planar CT images enable accurate and rapid probe placement. CT is also the modality of choice for post-ablation follow-up evaluations (Figure 1). Immediately after ablation therapy, targeted lesions are replaced by dense opacity surrounded by GGO (5). GGO margins of <3 mm have been associated with local treatment failure. During the early post-ablation period (<2 months), central dense opacity and surrounding GGO serves as the new “baseline”, and any increase in lesion size during follow-up should be considered local progression. However, morphologic evolution occurs in ablation zones and its extent depends on the thermal ablation methods used, for example, RFA-treated lesions show a relatively slow rate of involution, with a 40% decrease in size at 15 months after treatment, whereas cryoablated lesions show more rapid involution on follow-up CT images (6). PET/CT may be helpful for differentiating morphologic evolution and local recurrence, but early PET/CT within 3 months of ablation can be confounded by inflammatory changes.
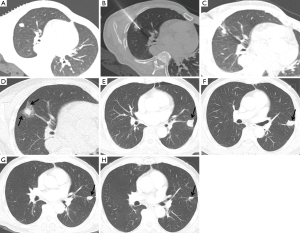
Thermal ablation has been shown to be both safe and effective for the treatment of primary and secondary pulmonary malignancies in nonsurgical candidates. For stage I NSCLC treated by RFA, 1- and 5-year overall survival (OS) rates have been reported to be 78% and 27%, respectively (7). However, more recent studies have reported better outcomes [2-year OS rate of 69.8% (8), 3-year OS rate of 74–79% (9,10), and 5-year OS rate of 58.1% (11)], which could be explained by multiple factors, including technical improvements, better patient selection, and the use of molecular-targeted therapies. Long-term outcome data for the treatment of NSCLC by MWA or cryoablation are limited; 1- and 3-year OS rates of 75% and 29.2% after MWA were reported for 48 patients with NSCLC of various stages (12), and 2- and 5-year OS rates of 88% and 67.8% were reported after cryoablation in patients with stage I NSCLC (13).
Thermal ablation has a role in the setting of advanced disease or as salvage therapy. In one study, after RFA for recurrent NSCLC (n=51) initially treated surgically, 1- and 5-year OS rates were 97.7% and 55.7% (14), and in patients with stage III or IV NSCLC, longer median OS was found for patients treated with RFA and chemotherapy than for those that received chemotherapy alone (median OS, 42 vs. 29 months, respectively; P<0.03) (15).
Thermal ablation plays a role in the management of metastatic disease in selected patients with limited disease burden. In general, this population includes patients with up to four lesions per lung and with lesions <3.5 cm (16). In the largest series conducted to date on pulmonary metastases (566 patients with 1,037 metastases) treated by RFA, median OS was 62 months and 1- and 5-year OS rates were 92.4% and 51.5%, respectively (17); multivariable analysis showed that primary disease other than colorectum and kidney, disease-free interval ≤1 year, a tumor size >2 cm, and number of metastases ≥3 were associated with poor OS (17).
There are limited comparative studies of thermal ablation with surgery and SBRT. It has been reported local recurrence was higher for RFA, and OSs were similar for RFA (n=8) and surgery (n=14) in stage I NSCLC (18). In another study, though RFA-treated stage I and II NSCLC patients were significantly older than those treated surgically, no significant difference in OS was observed (15). An analysis conducted on the National Cancer Institute’s Surveillance, Epidemiology, and End Results database (n=1,897) found OS and cancer specific survival were not significantly different between propensity score matched NSCLC patients treated surgically or by ablation (19). In a comparative study between RFA and SBRT in patients (n=48 and 47, respectively) with NSCLC of <5 cm, 3-year local control and OS rates were similar (20).
For pulmonary metastasis, RFA has been shown to be comparable to surgical resection with reported 5-year OS rates of 27–70% (21). Furthermore, because thermal ablation techniques can be administered repeatedly without substantially diminishing pulmonary function (8), these techniques have particular benefit in the setting of recurrent or residual disease.
Local progression is the main obstacle of thermal ablation, and local failure is associated with a large tumor size (>2 cm) and insufficient ablation margin (17,22). Additional biomarkers (e.g., Ki-67, KRAS, and EGFR), inflammatory cytokines, and immune markers, and imaging findings have been the focus of studies aimed at the early prediction of local recurrence and treatment response (23,24).
In summary, thermal ablation has been shown to be safe and effective for the treatment of primary and secondary lung malignancies in nonsurgical candidates, although there has been no large randomized study comparing ablation to surgery or SBRT. The benefits of these techniques include the preservation of more lung tissue than is possible by surgical resection and a reduction in morbidity as compared with surgery. These benefits are particularly important for lung cancer patients with high levels of comorbidities or limited pulmonary functional reserve due to chronic obstructive pulmonary disease. Lesion size is the main determinant of treatment success and survival. Future studies are required to refine patient selection, procedural techniques, and the assessment of local recurrence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mouli SK, Kurilova I, Sofocleous CT, et al. The Role of Percutaneous Image-Guided Thermal Ablation for the Treatment of Pulmonary Malignancies. AJR Am J Roentgenol 2017;209:740-51. [Crossref] [PubMed]
- Ihara H, Gobara H, Hiraki T, et al. Radiofrequency Ablation of Lung Tumors Using a Multitined Expandable Electrode: Impact of the Electrode Array Diameter on Local Tumor Progression. J Vasc Interv Radiol 2016;27:87-95. [Crossref] [PubMed]
- Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol: J Vasc Interv Radiol 2001;12:1135-48. [Crossref] [PubMed]
- de Baère T, Palussière J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 2006;240:587-96. [Crossref] [PubMed]
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 2012;32:947-69. [Crossref] [PubMed]
- Ito N, Nakatsuka S, Inoue M, et al. Computed tomographic appearance of lung tumors treated with percutaneous cryoablation. J Vasc Interv Radiol 2012;23:1043-52. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Hiraki T, Gobara H, Mimura H, et al. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:24-30. [Crossref] [PubMed]
- Liu B, Liu L, Hu M, et al. Percutaneous radiofrequency ablation for medically inoperable patients with clinical stage I non-small cell lung cancer. Thorac Cancer 2015;6:327-33. [Crossref] [PubMed]
- Palussière J, Marcet B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011;34:989-97. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. [Crossref] [PubMed]
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Kodama H, Yamakado K, Takaki H, et al. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc Intervent Radiol 2012;35:563-9. [Crossref] [PubMed]
- Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol 2012;35:343-50. [Crossref] [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 2012;81:395-9. [Crossref] [PubMed]
- Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol 2014;25:1-9.e1. [Crossref] [PubMed]
- Ochiai S, Yamakado K, Kodama H, et al. Comparison of therapeutic results from radiofrequency ablation and stereotactic body radiotherapy in solitary lung tumors measuring 5 cm or smaller. Int J Clin Oncol 2015;20:499-507. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 2009;32:478-83. [Crossref] [PubMed]
- Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol 2013;20 Suppl 3:S676-83. [Crossref] [PubMed]
- Schneider T, Sevko A, Heussel CP, et al. Serum inflammatory factors and circulating immunosuppressive cells are predictive markers for efficacy of radiofrequency ablation in non-small-cell lung cancer. Clin Exp Immunol 2015;180:467-74. [Crossref] [PubMed]


