Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for Chinese patients with squamous cell carcinoma of lung harboring EGFR mutation
Introduction
The presence of activating epidermal growth factor receptor (EGFR) mutation was associated with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) treatment response in patients with advanced non-small-cell lung cancer (NSCLC) (1). Recent phase III clinical studies of advanced NSCLC have demonstrated that EGFR mutations are the most effective predictor of clinical outcome in response to EGFR-TKIs in first-line treatment. The response rate ranged from 70% to 85% in EGFR mutant patients, with the progression-free survival (PFS) of 8 to 13 months (2-5). However, EGFR mutations occur almost exclusively in lung adenocarcinoma and the majority data on EGFR-TKIs sensitivity was derived from this histology. Due to the low mutation rate in squamous cell carcinoma of lung (SQCC) (6), EGFR mutation testing is not routinely recommended for SQCC in National Comprehensive Cancer Network (NCCN) guideline. Until now, no molecular targeted agents have been specifically developed for SQCC treatment.
EGFR mutation rate varied in previous reports, ranging from 1% to 15% (7-10). Several relative large series studies of surgically-resected SQCC found no EGFR mutations (11,12). But a meta-analysis from Asian reported that EGFR mutation rate was 10% in SQCC (13). As is known, the frequency of EGFR mutation is much higher in Asian population (14). Whether ethnic difference also existed in Chinese SQCC patients is not yet known. Another pool analysis from Japan demonstrated that the PFS of EGFR-TKIs in EGFR mutant SQCC was only 3 months, with the response rate of 30% (15). The research indicated that there was a subgroup of NSCLC patients harboring EGFR mutations with poor efficacy after EGFR-TKIs treatments, which was different from that of adenocarcinoma patients.
In the traditional diagnosis of NSCLC subtypes, no detailed pathologic analysis was provided. Poorly differentiated carcinoma might appear indistinguishable with morphologic diagnosis only (16). Great efforts had been made on distinguishing subtypes of NSCLC through immunohistochemical biomarkers these years (17-20). In 2011, a new multidisciplinary classification of lung adenocarcinoma has been developed, taking into account histologic, molecular, and radiologic features, as well as prognostic and predictive information for treatment selection (21). Several studies have already demonstrated that immunohistochemistry (IHC) of TTF-1 and P63 can effectively identify the tumor cell type in samples of NSCLC (17,18). Recently, another research have shown that combined with Napsin A, P63 and TTF-1 could be sufficient to reliably sub-classify poorly differentiated NSCLC (22). However, in pre-IHC era, the lack of precision in morphologic diagnosis of NSCLC subtypes might account for the variability of reported EGFR mutation rate in SQCC.
Whether EGFR mutations do arise in SQCC was still a controversial topic. And the efficacy of EGFR-TKI was still disputable in EGFR mutant SQCC. Here, we performed a retrospective study to estimate the EGFR mutation rate in IHC-verified SQCC and to evaluate the efficacy of EGFR-TKIs in Chinese patients with advanced SQCC harboring EGFR mutations.
Patients and materials
Study cohorts
Two cohorts of patients with SQCC were enrolled onto the research. All patients were treated at the Cancer Center of Sun Yat-Sen University (Guangzhou, China) from 1 January 2004 to 31 August 2012.
The first cohorts consisted of 146 consecutive patients with diagnosis of SQCC who had received radical resection from 1 January 2008 to 1 January 2012. A representative formalin-fixed, paraffin-embedded (FFPE) tumor block was collected for IHC reassessment and EGFR mutation analysis.
The second cohort included 67 patients with advanced SQCC who had received EGFR-TKIs (Gefitinib or Erlotinib) treatment in the course of disease from January 2004 to 31 August 2012. All the patients were diagnosed with advanced lung SQCC by bronchofiberscope biopsy or percutaneous lung biopsy. Only 63 patients had adequate specimens for further analysis. The FFPE tumor blocks of the 63 patients were also collected and subjected to IHC reassessment for pathological diagnosis, as described later. EGFR mutation analysis was also performed on this cohort of patients. The efficacy of EGFR-TKIs was all available.
The basic clinical data of the two cohorts of patients was collected, including age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage, smoking status. In the second cohort, the response evaluation of patients with EGFR-TKIs was recorded. The prognosis of the patients in this cohort was retrospectively analyzed, including response rate and PFS.
The study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center (Guangzhou, China). All the patients had provided written informed consent before samples were collected.
EGFR mutation analysis
The FFPE tumor blocks were cut into 5 μm consecutive sections for DNA extraction. The EGFR Scorpion ARMS Kit (DxS Ltd, Manchester, UK) was used to detect EGFR mutations by real-time PCR, which enabled to detect the low-level mutant DNA in the background of wild-type DNA based on the allele-specific and real-time PCR technologies. 2 ng DNA was added to each 25 µL assay reaction in 96-well plate. The plate was sealed and loaded into Stratagene MX3005P real-time PCR system (Agilent Technologies, Santa Clara, Canada). Obtained data was analyzed using MxPro v4.0 software (Agilent Technologies, Santa Clara, Canada).
IHC analyses
IHC staining was performed using mouse monoclonal anti-human antibodies for reconfirming the pathological diagnosis, including P63 (DAKO, 1:80), TTF-1 (DAKO, 1:600) and Napsin A (DAKO, 1:200). Sections with 5-μm-thick were cut from the FFPE and then routinely deparaffinized and rehydrated. For antigen retrieval, slides were heated in a microwave oven for 30 minutes in citrate buffer solution (pH=7.4) and cooled slowly at room temperature for 20 minutes. After blocking the activity of endogenous peroxidase with 3% hydrogen peroxide for 8 minutes, the sections were treated with primary antibodies and incubated for 12 hours. Subsequently, the slides were rinsed in PBS three times and incubated in biotinylated secondary antibodies. After incubation, slides were washed again with PBS and then visualized using diaminobenzidine. Finally, Mayer’s hematoxylin was used to counterstain the sections, which were then dehydrated and mounted.
P63-diffuse/TTF-1-negative profile supported SQCC. The cases with diffuse P63 and weak/focal co-expression of TTF-1 were further confirmed as SQCC by negative Napsin A. The profiles that supported adenocarcinoma were TTF-1-positive and P63-negative. TTF-1 and P63 double-negative profile was interpreted as indeterminate but favoring adenocarcinoma because negative P63 is highly unusual for SQCC, whereas TTF-1-negative adenocarcinoma art not uncommon. Double-negative carcinomas were further evaluated with Napsin A. Reactivity for P63 and TTF-1 in distinct cell population generally supported biphenotypic differentiation, such as adenosquamous carcinoma. Two pathologists who don’t know the information of the patients were asked to independently assess the expression. The reassessment process was shown in (Figure 1).
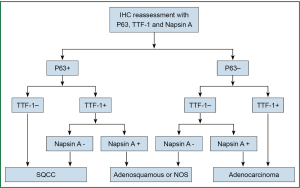
Statistical analysis
The EGFR mutation rate in lung SQCC was analyzed in the first cohort. In the second cohort, PFS with EGFR-TKIs was calculated from the beginning receiving TKIs to the time of disease progression. Patients who had not progressed at the time of statistical analysis were censored at the time of last follow-up (31 August 2012). Univariate analysis by log-rank test was conducted on PFS to evaluate the effects of clinical factors relating to prognosis. Multivariate analysis was performed with Cox regression analysis. And chi-square test was used for response rate and disease control rate analysis. Survival analysis was depicted by Kaplan-Meier method. A P value <0.05 used to denote statistical significant, and all reported P values were two sided. All statistical analyses were performed with SPSS 16.0.
Results
Patient characteristics
In the first cohort, of the 146 patients with diagnosis of SQCC after radical resection, 129 patients (88.4%) were male and 17 female (11.6%), with the median age 59 years old (range: 30-81 years old). All the patients were stage I to IIIA.
In the second cohort, 63 patients with advanced lung SQCC were retrospectively analyzed, including 50 male (79.4%) and 13 female (20.6%). The median age was 54 years (range: 23-75 years). All the patients received Erlotinib (n=33) or Gefitinib (n=30) as second or third line treatment. The basic clinical characteristics of the two cohorts are listed in (Table 1).
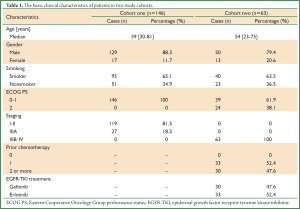
Full Table
IHC reassessment and EGFR mutation analysis
All the patients in the two cohorts had adequate specimens for IHC reassessment. The pathologic diagnosis of all the patients were verified to be SQCC with P63+/TTF-1+/-/Napsin A– (Figure 2).
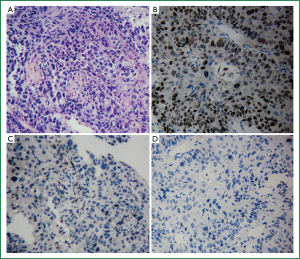
In cohort one, EGFR mutations were detected in only 3 patients (2.0%). All the 3 patients were L858R mutations. No other mutations were detected. Hence, the EGFR mutation rate in Chinese lung SQCC was only 2.0%.
In cohort two, all 63 patients had additional samples for EGFR mutations analysis. EGFR mutations were detected in 15 patients (23.8%), including exon 19 deletions (n=13) and L858R mutation (n=2). The other 48 patients were EGFR wild-type.
Efficacy of EGFR-TKIs in verified advanced lung SQCC
In the 63 patients of verified SQCC, 5 patients achieved partial response (PR), among whom 1 patient received Gefitinib and 4 patients received Erlotinib. 25 achieved stable disease (SD), including 9 with Gefitinib and 21 with Erlotinib. The response rate and disease control rate were 7.9% and 47.6%, respectively.
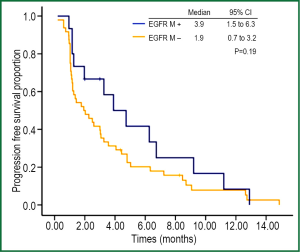
At the end of data cut off, 4 patients (all SD) had not experienced progression at the last follow-up (31 Aug 2012). The median PFS of all patients was 2.5 months (95% CI: 1.3-3.7 months). Results of univariate analysis for PFS are shown in (Table 2). No factor was correlated significantly with PFS. Although the response rate of EGFR-mutant patients was better than that of EGFR wild-type patients (26.7% vs. 2.1%, P=0.002), the disease control rate between the two groups was not significantly different (66.7% vs. 41.7%, P=0.09) (Table 3). The PFS of EGFR-mutant patients were numerically longer than that of EGFR wild-type patients (3.9 vs. 1.9 months, respectively). No significant difference was observed between these two groups of patients (P=0.19) (Figure 3).

Full Table

Full Table
Discussion
Although the incidence of lung SQCC is decreasing as a consequence of changes in tobacco consumption habits (23,24), it is still the second most common type of NSCLC (25). Encouraging new target agents have provided great benefit to patients with adenocarcinoma, but, unfortunately, there was no effective targeted therapy for lung SQCC to date. EGFR-TKIs were now recommended as first-line treatment for NSCLC patients with sensitive EGFR mutation, which was mainly in lung adenocarcinoma. The frequency of EGFR mutation in lung SQCC varied in previous reports and in different ethics, ranging from 1% to 15% (7-10). In the China Edition of NCCN guideline, EGFR mutation detecting is recommended for SQCC basing on a meta-analysis. Hence, discrepancy existed in the guideline recommendation in different ethnicity.
Recently Natasha R. et al. retrospectively analyzed 95 biomarker-verified SQCC and reported that EGFR mutations do not occur in pure SQCC, and occasional detection of EGFR mutations in samples of SQCC was due to diagnosis of adenosquamous carcinoma or adenocarcinoma (26). However, another similar study was conducted by Miyamae et al. (9), demonstrated that EGFR mutations were detected in 3.4% among 87 validated lung SQCC specimens. To prove this controversial topic in Chinese population, we reassessed the pathologic diagnosis of the specimens and evaluated the EGFR mutation rate in Chinese squamous cell lung cancer patients in our study, which was the first time of EGFR mutation rate screening in Chinese squamous cell lung cancer with large cohort of patients. All the patients in cohort one had received operation and the large specimens were used for testing. All the patients were validated with IHC to be true SQCC. Only 3 patients possessed EGFR mutation. Hence, EGFR mutation did exist in true SQCC, with extremely low mutation rate of 2.0%, which was much lower than the previous reports of 10% (13). Recently, another research about comprehensive genomic analysis of lung SQCC reported 2 patients with EGFR mutation from 178 verified SQCC patients (27). The result was consistent with our study. It seemed that in SQCC patients, the frequency of EGFR mutation was similar in different ethnicity.
Although EGFR mutation was rare in lung SQCC, it was reported that the PFS of EGFR-TKIs in EGFR mutant SQCC was 3 months, with response rate and disease control rate of 30% and 70% respectively in a pool analysis (12). It seemed that EGFR-mutant SQCC could still obtain survival benefit from target therapy. However, the efficacy of EGFR-TKIs in EGFR mutant and EGFR wild-type SQCC patients has not yet been fully compared before. Here, we retrospectively analyzed 63 advanced SQCC patients who had received EGFR-TKIs treatment, including 15 EGFR mutant patients and 48 EGFR wild-type patients. The response rate in EGFR mutant patients was much higher (26.7% vs. 2.1%, P=0.002), but the disease control rate was not significantly different between the two groups (66.7% vs. 41.7%, P=0.09). The response rate and disease control rate in EGFR mutant patients were consistent with the report of pool analysis. The PFS was numerically longer in EGFR mutant patients (3.9 vs. 1.9 months), but no significant difference was observed (P=0.19), which might be caused by the relative small sample size. The results indicated that the efficacy of EGFR-TKIs in SQCC was limited, which was much poorer with historical comparison of EGFR mutant in Asian adenocarcinoma of 30% (14). There were few treatment options for patients with advanced lung SQCC. Platinum-based doublet chemotherapy was still the first choice. EGFR-TKIs could still be taken as a salvage treatment.
Interestingly, in the 48 wild-type EGFR patients, 1 achieved PR in response to erlotinib and another 19 patients achieve SD. The sensitivity of EGFR mutation tests was suspected to be one of the possible reasons for the response of EGFR-TKIs in patients without detectable EGFR mutations. In our study, sensitive method was used to detect EGFR mutation. Besides, it had been reported that EGFR mutations were evenly distributed in lung tumors. In the heterogeneity of EGFR mutation analysis by Yatabe et al. identical EGFR mutations were found throughout individual tumors in a trans-sectional analysis and no discordant mutation patterns were detected among paired primary and metastatic site samples (28). The study indicated that EGFR mutation detection with different sizes or location merely affected the results. Another possible reason is that EGFR-TKIs might target additional pathways other than EGFR mutations, which still needed further study to validate.
The BR.21 (NCT00036647) and TRUST (NCT 00949910) study had proved the efficacy and safety of erlotinib in second-line or third-line treatment comparing with placebo (29,30). Recently, the Tailor study (NCT00637910) indicated that previously treated wild-type EGFR patients could not obtain PFS benefit from the second-line treatment of erlotinib when comparing with docetaxel (31). However, the subgroup analysis in SQCC patients showed similar PFS.
In the 15 patients harboring EGFR mutations, 5 patients failed to response to EGFR-TKIs treatment. According to pervious study, the presence of T790M mutation might account for the lower efficacy (15,32). Recently, a comprehensive genomic analysis of lung SQCC has demonstrated the complex genomic alternations on the core cellular pathway of SQCC. The PI3K/RTK/RAS signaling pathway possessed 69% of alternation, which might affect the efficacy of EGFR-TKIs (27). Besides, coexistence of PI3K mutation and EGFR mutation has been reported (33), which might also help to explain the poor efficacy of TKI in SQCC. Hence, it is suggested that combined analysis of KRAS, PI3KCA, MET and non-sensitizing EGFR mutation was necessary before treatment.
There are several major limitations of our study. First, this is a retrospective study. All the data were collected retrospectively. And the frequency of EGFR mutation rate of SQCC was from the early stage patients. Second, small sample size in cohort two might affect the statistically analysis. There were only 66 patients treated with EGFR TKIs analysis, and only 15 patients possessed EGFR mutation. Thirdly, due to the small specimens, we didn’t have adequate samples for further molecular analysis.
In conclusion, lung SQCC validated with new WHO criteria did possess EGFR mutation, but the frequency of EGFR mutation in Chinese patients was only 2.0%, which was much lower than the previous reports. In EGFR mutant lung SQCC patients, the PFS was numerically longer than that of EGFR wild-type patients. The low mutation rate and limited efficacy don’t justify routine test for all SQCC specimens. However, EGFR-TKIs could be taken as a salvage treatment for advance lung SQCC patients. Due to the small sample size of retrospective study, prospective studies with large cohort are warranted in the future.
Acknowledgements
This work was supported in part by the grant from the National High Technology Research and Development Program of China (2012AA02A502), the grants from the Nature Science Foundation of China (81372502, 81201917) and the Specialized Research Fund for the Doctoral Program of Higher Education (20120171120116).
Disclosure: The authors declare no conflict of interest
References
- Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 2010;10:760-74. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Forbes SA, Bhamra G, Bamford S, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet 2008;Chapter 10:Unit 10.11.
- Park SH, Ha SY, Lee JI, et al. Epidermal growth factor receptor mutations and the clinical outcome in male smokers with squamous cell carcinoma of lung. J Korean Med Sci 2009;24:448-52. [PubMed]
- Huang SF, Liu HP, Li LH, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res 2004;10:8195-203. [PubMed]
- Miyamae Y, Shimizu K, Hirato J, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep 2011;25:921-8. [PubMed]
- Ohtsuka K, Ohnishi H, Fujiwara M, et al. Abnormalities of epidermal growth factor receptor in lung squamous-cell carcinomas, adenosquamous carcinomas, and large-cell carcinomas: tyrosine kinase domain mutations are not rare in tumors with an adenocarcinoma component. Cancer 2007;109:741-50. [PubMed]
- Sugio K, Uramoto H, Ono K, et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer 2006;94:896-903. [PubMed]
- Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857-65. [PubMed]
- Wu YL, Zhong WZ, Li LY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007;2:430-9. [PubMed]
- Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer -- search and destroy. Eur J Cancer 2006;42:17-23. [PubMed]
- Shukuya T, Takahashi T, Kaira R, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci 2011;102:1032-7. [PubMed]
- Travis WD, Rekhtman N, Riley GJ, et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: a paradigm shift. J Thorac Oncol 2010;5:411-4. [PubMed]
- Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol 2011;24:1348-59. [PubMed]
- Pelosi G, Rossi G, Bianchi F, et al. Immunhistochemistry by means of widely agreed-upon markers (cytokeratins 5/6 and 7, p63, thyroid transcription factor-1, and vimentin) on small biopsies of non-small cell lung cancer effectively parallels the corresponding profiling and eventual diagnoses on surgical specimens. J Thorac Oncol 2011;6:1039-49. [PubMed]
- Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol 2011;35:15-25. [PubMed]
- Righi L, Graziano P, Fornari A, et al. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology: a retrospective study of 103 cases with surgical correlation. Cancer 2011;117:3416-23. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Noh S, Shim H. Optimal combination of immunohistochemical markers for subclassification of non-small cell lung carcinomas: A tissue microarray study of poorly differentiated areas. Lung Cancer 2012;76:51-5. [PubMed]
- Ginsberg MS, Grewal RK, Heelan RT. Lung cancer. Radiol Clin North Am 2007;45:21-43. [PubMed]
- Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41:245-58. [PubMed]
- Wahbah M, Boroumand N, Castro C, et al. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol 2007;11:89-96. [PubMed]
- Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012;18:1167-76. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [PubMed]
- Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972-7. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Laack E, Schneider C, Gutjahr T, et al. Association between different potential predictive markers from TRUST, a trial of erlotinib in non-small cell lung cancer (NSCLC). J Clin Oncol 2007;25:abstr 7651.
- Garassino MC, Martelli O, Bettini A, et al. TAILOR: a phase III trial comparing erlotinib versus docetaxel as second-line treatment of NSCLC patients with wild-type EGFR. J Clin Oncol 2012;30:abstr LBA7501.
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [PubMed]
- Ludovini V, Bianconi F, Pistola L, et al. Optimization of patient selection for EGFR-TKIs in advanced non-small cell lung cancer by combined analysis of KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer Chemother Pharmacol 2012;69:1289-99. [PubMed]


