What is difficult about doing video-assisted thoracic surgery (VATS)? A retrospective study comparing VATS anatomical resection and conversion to thoracotomy for lung cancer in a university-based hospital
Introduction
Video-assisted thoracic surgery (VATS) lobectomy is an acceptable alternative to thoracotomy lobectomy for early-stage lung cancer (1,2). However, conversion to thoracotomy is sometimes inevitable due to intraoperative massive bleeding, pleural adhesion, enlarged or fibrocalcified lymph node, or anatomic variations (3-5). The conversion rate varies from 4% to 23% (4-8) because of diverse study periods or populations but is usually higher in the hospital where smaller cases of surgical resection are performed or due to surgeons being in the learning curve (6,7). Moreover, this presents a large amount of stress because nearly half of conversions to thoracotomy are performed in emergency situations (7-9).
Therefore, many reports have tried to analyze predictors of conversion to thoracotomy. These include tumors more than 3 cm, calcification score, age, or tumor location (4-8). However, it is difficult to accept the results as they are. This is because the number of operations is variable in each hospital, and the troubles occurring at hospitals that have just started using the VATS lobectomy procedure will be different from those seen at large and experienced hospitals. We investigated the medical records of lobectomy in our hospital with a short history of VATS lobectomy and analyzed the factors affecting the conversion to thoracotomy. Thus, this can be a practical guideline for hospitals still in the VATS learning curve.
So, the aim of this study was to analyze causes and clinical outcomes of conversion to thoracotomy during VATS anatomical resection for patients with non-small cell lung cancer.
Methods
Patients
Lung resections performed in the Chungbuk National University Hospital from January 2013 to July 2016 were analyzed retrospectively. Inclusion criteria were as follows: (I) non-small cell lung cancer; (II) curative surgery with R0 resection and (III) anatomical resection with lobectomy or segmentectomy. The exclusion criteria were as follows: (I) a history of neoadjuvant therapy that could influence thoracotomy conversion; (II) benign lung disease; (III) incomplete resection with R1 or R2 resection; (IV) multiple pulmonary malignancies and (V) non-anatomical surgery with wedge resection. This study was reviewed and approved by the Institutional Review Board of Chungbuk National University Hospital (2017-03-005-001).
Basic demographics and medical factors included age, gender, previous illness history, American Society of Anesthesiologists (ASA) score, height, weight, body mass index (BMI), and pulmonary functions. Preoperative imaging studies including chest computed tomography (CT) or positron emission tomography-computed tomography (PET-CT), surgical and pathologic data were analyzed. In particular, we investigated cases with suspected inflammation or infectious status showing increased lymph node size by chest CT or fluorodeoxyglucose (FDG) uptake by PET-CT in addition to definite metastatic lesions
Documentation of thoracotomy conversion and grouping
The group “conversion” was defined as (I) surgery initiated with VATS such as, 3 or 4 ports for VATS instruments were made; (II) VATS surgery was continued to perform anatomical lung resection such as, segmentectomy or lobectomy with mediastinal lymph node dissection; (III) VATS stopped due to a prominent cause and thoracotomy added for continuing anatomical lung resection. The group “VATS” was defined as surgery initiated with VATS and finished without conversion to thoracotomy.
Study design and statistics
The primary outcome was conversion to thoracotomy. The study population was divided into three categories as follows: (I) patients whose surgery began with thoracotomy; (II) “conversion” patients whose initiated surgery began with VATS but resulted in thoracotomy conversion; (III) “VATS” patients whose surgery began and finished with VATS. The two groups, “conversion” and “VATS” were compared on preoperative basal characteristics, functional factors, radiologic findings and clinical outcomes. Descriptive statistics were used to describe patient demographics and outcomes. Categorical data were expressed as numbers and proportions. Continuous data were expressed as means with standard deviations. Chi-square or Fisher’s exact tests were used to compare categorical data, and t-tests were used for continuous variables. Logistic regression methods were adapted to discover significant factors predicting conversion to thoracotomy. A P value less than 0.05 was considered significant. Statistical analysis was performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina, United States) and R 2.13.2 (Vienna, Austria; http://www.R-project.org/).
Results
Basic demographics and preoperative work-up
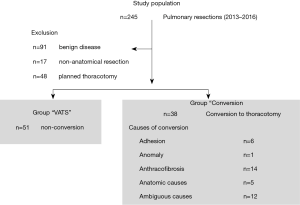
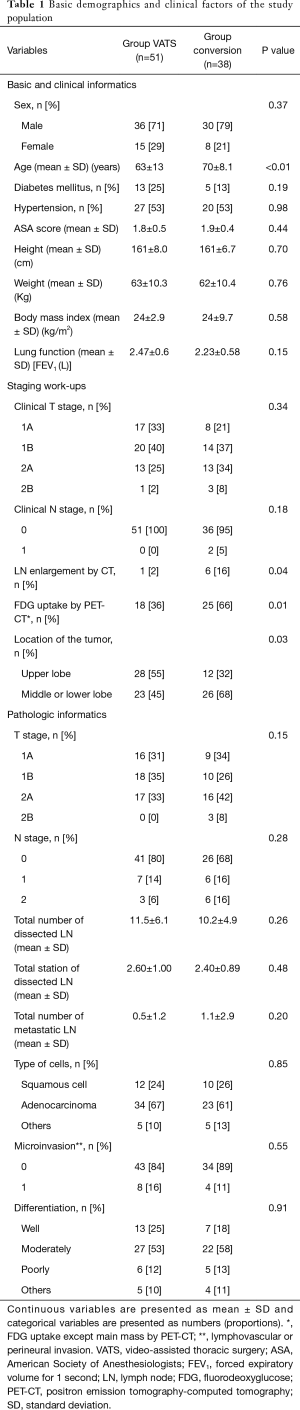
A total of 245 pulmonary resections were performed during the study period. Of these, 91 (benign lung disease) and 17 (wedge resection) were excluded from the study. Of the 137 patients, 89 were included in the study, except for 48 patients who finished surgery and underwent thoracotomy without VATS surgery. The VATS group consisted of 51 cases and the conversion group included 38 patients (Figure 1). In both groups, the proportion of males was over 70%, and there were no statistical differences between genders with respect to previous illness history, functional status, and anthropometric data. However, the conversion group (mean age, 70 years) was older than the VATS group (mean age, 63 years) (P<0.01). There was no difference in clinical stage between groups. Detailed information is reported in Table 1. However, no obvious metastasis, the presence of enlarged lymph nodes or FDG lesion uptake was significantly higher in the conversion group (P=0.04, P=0.01). Tumor location was also different between groups. The conversion group had more middle or lower lesions (upper: 32%, middle and lower: 68%) but the VATS group (upper: 55%, middle and lower: 45%) did not (P=0.03). Pathologic stage in the conversion group (stage I: 50% vs. stage II: 50%) was higher than that in the VATS group (stage I: 66% vs. stage II: 34%), but this was not statistically significant. There were no significant differences in the total number of resected lymph nodes, total number of stations, number of metastatic lymph nodes, cell types or differentiation, or microinvasion of lymphatics, microvessels, or pleura.
Full table
Perioperative outcomes
Thoracotomy conversions
The conversion group (n=38) was composed of 28% of total lung cancer surgery and 43% of VATS attempts. The conversion to thoracotomy was performed for the following reasons. Anthracofibrosis caused 14 cases of uncontrollable bleeding or problems with vessel dissection, 6 cases of severe pleural adhesions, and 6 cases of anatomic problems including vascular anomaly. In addition, there were 12 cases where it was determined that thoracoscopic surgery was no longer feasible due to technical problems. Univariate analysis was performed to investigate determining factors that predicted conversion to thoracotomy. Significant factors were as follows: age (P<0.01), enlarged lymph node by chest CT (P=0.04), lesions with FDG uptake except the main mass or prominent lymph node metastasis (P<0.01), and tumor location (P=0.03). Multivariate analysis revealed that age (P=0.049) and tumor location (P=0.039) were significant factors involved in conversion to thoracotomy (Table 2).

Full table
Perioperative outcomes
Time of operation was longer in the conversion group (222 min) compared to the VATS group (160 min) (P<0.01). However other factors were not significantly different between groups, although trends for estimated blood loss, chest tube drainage, length of hospital stay, and rate of major complications were lower in the VATS group (P=0.05, 0.07, 0.35 and 0.37, respectively) (Table 3).

Full table
Discussion
Our results showed a relatively higher conversion rate for patients in hospitals with a short history of VATS surgery. There is, of course, no reason to simply compare numerically the results of different institutions because there is room for selection bias since this was a prospective study. For example, conversion rate may change depending on how indications for VATS surgery are set. We performed VATS anatomical resection in 89 (65%) out of 137 patients with lung cancer and recorded a conversion rate of 43%. In general, the conversion rate increases during staff learning curve, but this can be overcome as a certain amount of experience is accumulated (10,11). Although the conversion rate for VATS anatomical surgeries in our hospital has increased temporarily (30% in 2013 to 48% in 2014), this has improved with accumulated experience (48% in 2015 to 39% in 2016). The conversion rate is expected to decline further if experience is set to accommodate emergent situations and appropriate surgical indications in the future.
The clinical outcomes between the conversion and VATS groups were not statistically different except for operation time. Although the clinical outcome of the conversion group looks inferior, it is difficult to predict a statistical difference in the future because clinical outcomes, including the conversion rate, improve as experience increases (10). Some studies have shown that there are no differences in clinical outcome between these two groups (4-6), but others have shown there are (3,7). However, it is clear that many conversions to thoracotomy are required in emergency situations (6,7) and this may produce unexpected results for surgeons and patients. So, the effort to predict and avoid the risk of conversion seems to be necessary.
Age and tumor location were the main causes of conversion to thoracotomy in this study. As age increased, systolic pressure in the pulmonary vessels increased, but the medial collagen content of vessel walls decreased, resulting in less extensible changes (12,13). So, the pulmonary blood vessels of elderly patients are much more vulnerable to the same stresses as those of younger patients, and it is likely to result in blood vessel rupture and the like during surgery. In addition, due to the characteristics of South Korea, which is a pulmonary tuberculosis disease country, anthracofibrosis around the bronchus increases with age (14) making pulmonary vascular dissection difficult. In other words, elderly patients are more likely to experience unexpected thoracotomy conversion due to vascular damage and subsequent bleeding during pulmonary vascular dissection. The change in conversion rate according to lung cancer location is expected to be further investigated. In some studies, the conversion rate of thoracotomy in the upper lobe was higher than that in the lower lobe, but in this study, the conversion rate for the middle and lower lobes was higher than that for the upper lobe. There is a possibility that selection bias has intervened and we need to isolate the cause via future large-scale studies.
This study has the following limitations. First, cases with surgical interventions were done by different surgeons, and due to the nature of the retrospective study, it was not possible to define conversion to thoracotomy with unified criteria. However, we think that it is an inevitable part of this study which was not performed prospectively. Moreover, surgeons with different careers were included in the study. This may correct the technical differences between surgeons, but can also result in selection bias, so it is necessary to validate this study through large-scale studies. Second, since this study occurred in the context of a university-based hospital with surgeons still in a learning curve period, it is unreasonable to apply our results to large centers. However, we think that our data can be used as a reference when starting a VATS anatomical resection for lung cancer in a similar hospital.
In conclusion, hospitals currently in learning curves that attempt VATS anatomical resection in lung cancer patients may experience a relatively high thoracotomy conversion rate which may increase in elderly patients or cases of middle or lower lobe tumors. There was no difference in clinical outcomes between groups except in the time of operation, but unexpected results could occur. Therefore, it is recommended that appropriate preparation is required when conversion to thoracotomy is expected.
Acknowledgements
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1C1B5015969).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by the Institutional Review Board of Chungbuk National University Hospital (No. 2017-03-005-001).
References
- Yang CF, Sun Z, Speicher PJ. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for Non-Small Cell Lung Cancer by Video-Assisted Thoracic Surgery: Effects of Cumulative Institutional Experience on Adequacy of Lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Byun CS, Lee S, Kim DJ, et al. Analysis of Unexpected Conversion to Thoracotomy During Thoracoscopic Lobectomy in Lung Cancer. Ann Thorac Surg 2015;100:968-73. [Crossref] [PubMed]
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Smith DE, Dietrich A, Nicolas M, et al. Conversion during thoracoscopic lobectomy: related factors and learning curve impact. Updates Surg 2015;67:427-32. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]
- Mazzella A, Olland A, Falcoz PE, et al. Video-assisted thoracoscopic lobectomy: which is the learning curve of an experienced consultant? J Thorac Dis 2016;8:2444-53. [Crossref] [PubMed]
- Yu WS, Lee CY, Lee S, et al. Trainees Can Safely Learn Video-Assisted Thoracic Surgery Lobectomy despite Limited Experience in Open Lobectomy. Korean J Thorac Cardiovasc Surg 2015;48:105-11. [Crossref] [PubMed]
- Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009;119:2663-70. [Crossref] [PubMed]
- Mackay EH, Banks J, Sykes B, et al. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax 1978;33:335-44. [Crossref] [PubMed]
- Park TY, Heo EY, Chung HS, et al. Prediction of Anthracofibrosis Based on Clinico-Radiographic Findings. Yonsei Med J 2017;58:355-61. [Crossref] [PubMed]


