Chemoradiotherapy for unresectable cases of thymic epithelial tumors: a retrospective study
Introduction
Thymic epithelial tumors (TETs) are rare, with an annual incidence of 1.3–3.2 per 100,000 person-years (1-3). They comprise thymoma and thymic carcinoma; these are distinguished by their pathological features, with thymic carcinoma accounting for 1–4% of TET cases. In thymoma, the development of CD4+/CD8+ double positive T-cells with preserved thymic function can lead to immunological complications, and patients with thymoma are sometimes complicated with myasthenia gravis, pure red cell aplasia, or hypogammaglobulinemia, even in the early stages. In contrast, thymic carcinoma can occasionally result in the loss of thymic function; thus, thymic carcinoma is usually diagnosed by symptoms derived from the invasion or metastasis of the disease and has a poor prognosis.
Currently, the standard management of TET remains controversial and supported by only a low level of evidence, which is a common problem with rare cancers (4). Patients with TET are usually treated with curative-intent surgery with a view to control the disease locally. When TET reaches Masaoka-Koga Stage IVB with hematogenous metastasis, palliative-intent chemotherapy is usually indicated. Effectiveness of adjuvant therapy is controversial (5,6). Neoadjuvant or induction chemotherapy/chemoradiotherapy has been evaluated in phase II trials (7-9), but there is no consensus over which regimen maximizes control of the diseases. For TET at Masaoka-Koga Stage IVB with lymphogeneous metastasis or circumstances where resection is not possible, along with the invasion of significant organs, curative-intent radiotherapy with or without chemotherapy, or surgery with multimodalities, is indicated. However, it is unclear whether the treatment is beneficial among patients with a locally advanced stage of TET (4). In the treatment of solid cancers, chemotherapy is known to sensitize radiotherapy and to enhance the control of micrometastases as a systemic control; however, concurrent radiotherapy with chemotherapy results in greater toxicity. To date, few data have been reported for TET.
The objective of the present study was to retrospectively evaluate clinical characteristics, prognosis, and prognostic factors for patients with unresectable, locally advanced thymoma and thymic carcinoma treated with radiotherapy combined with chemotherapy in our cancer center.
Methods
Patients and data acquisition
The databases at Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital (Tokyo, Japan), were retrospectively reviewed to identify patients treated with radiotherapy and chemotherapy for TETs identified between January 1, 1978 and October, 2016. The study was approved by the hospital’s institutional ethics board of Tokyo Metropolitan Komagome Hospital Review Board (No. 1872).
We collected data on the outcomes of 215 patients with TET, including 141 patients with thymoma and 74 patients with thymic carcinoma. Among these, 6 patients with thymoma and 14 with thymic carcinoma were treated with chemoradiotherapy without curative-intended surgery. Other possible malignant thoracic malignant tumors at diagnosis were excluded by an institutional pathologist (TH), who reviewed hematoxylin and eosin-stained samples on the basis of the 2015 World Health Organization classification of thymic cancers (10) and also assessed immunohistochemistry using a CD5 and/or CD117 (c-KIT). Staging was also determined using the Masaoka-Koga staging system, in accordance with the recommendation of the International Thymic Malignancies Interest Group (ITMIG) (11). We collated clinical factors such as age, staging, Eastern Cooperative Oncology Group (ECOG) Performance Status, cigarette smoking status, initial symptoms at diagnosis, the presence of extrathoracic metastasis, treatment modalities, and survival. Data regarding treatment modality included radiation dose, fractionation, chemotherapy regimen, and the concurrent or sequential strategy with radiotherapy and chemotherapy. Data were collected in accordance with the ITMIG Standard Definitions and Policies (12). Medical records and laboratory data for each patient were retrieved for the analysis and assessment of treatments for TET.
Treatment and evaluation
We identified 20 patients who were treated with radiotherapy in combination with sequential or concurrent chemotherapy for localized or locally advanced TET (at Masaoka-Koga Stage III or IVB with lymphatic dissemination). A response was defined as a patient who achieved a complete or partial response when whole lesion of the patient was assessed using the Response Evaluation Criteria in Solid Tumors criteria, version 1.1 (13).
Statistical analysis
The primary endpoint was to reveal clinical outcome in patients with locally advanced TET treated with radiotherapy in combination with sequential or concurrent chemotherapy. The Kaplan-Meier method was used to analyze survival time, defined as the period from the date of the initiation of treatment (definitive radiotherapy or induction chemotherapy) to the date either of death from any cause or of the final follow-up. Patients lost to follow-up were censored at the time of the last contact. Due to the retrospective nature of the data, these relevant points were chosen to reflect clinical practice. The Kaplan-Meier method was also used to estimate overall survival (OS), as well as 1-, 2-, and 5-year survival proportions. The log-rank test was used to identify prognostic factors for survival in univariate analysis. Histology (thymoma vs. thymic carcinoma), treatment modalities (sequential vs. concurrent), and chemotherapy regimens (platinum-containing vs. non-platinum-containing) were analyzed as variables that could determinate OS or progression-free survival (PFS). Multivariate analysis was not performed due to the small number of patients. Statistical analyses used R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
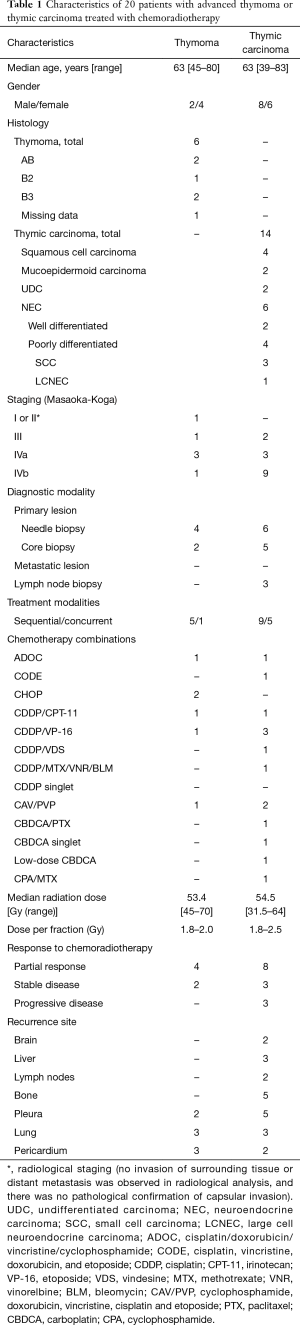
Of the 215 patients with TET retrieved from the database, comprising 141 patients with thymoma and 74 with thymic carcinoma, 20 (9.3%) were identified as having been treated with curative-intent radiotherapy with sequential or concurrent chemotherapy. Patient characteristics are presented in Table 1. These accounted for 4.3% (6/141) of the thymoma patients and 18.9% (14/74) of patients with thymic carcinoma. The median follow-up time was 35.4 months. The patients’ median age was 63 years (range, 39–83 years). At diagnosis, the disease stages were as follows: 1 (5%) at stage I or II (radiological diagnosis; no invasion to the surrounding tissues or metastasis was detected), 3 (15%) at stage III, 6 (30%) at stage IVa, and 10 (50%) at stage IVb. Histologic examination revealed that of the 14 patients with thymic carcinoma, 4 (29%) had squamous cell carcinoma, 6 (36%) had neuroendocrine carcinoma (NEC) [3 with small-cell carcinoma (SCC)], 1 with large-cell neuroendocrine (LCNEC) carcinoma, and 2 with a well-differentiated neuroendocrine tumor), 2 (14%) with mucoepidermoid carcinoma (14%), and 2 (14%) with undifferentiated carcinoma (UDC). Of the 6 patients with thymoma, 2 were type AB according to the WHO classification, 2 were type B3, and 1 was type B2; the histological data of the other patient with thymoma were missing. Autoimmune-related symptoms were observed in only two patients (myasthenia gravis and hypogammaglobulinemia, one patient each).
Full table
Clinical outcomes of radiotherapy with chemotherapy and factors affecting survival
Table 1 also presents the drug combinations and clinical outcomes of chemoradiotherapy. Of the six patients with thymoma, two (33%) received platinum-based doublet therapy (a combination of cisplatin or carboplatin and another cytotoxic reagent), and two were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone). The other two patients received ADOC (cisplatin, doxorubicin, vincristine, and cyclophosphamide) and CAV/PVP (cyclophosphamide, doxorubicin, vincristine, cisplatin, and etoposide. Of the 14 patients with thymic carcinoma, 6 (43%) received platinum-based doublet therapy, 2 (14%) received only carboplatin, and 4 (29%) received other platinum-containing regimens.
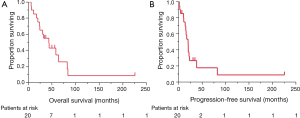
No patient achieved a complete response. The response rate for patients treated with platinum-containing chemotherapy (n=16) was 56%. The median OS time for all patients was 43.5 months (95% CI, 20.3–64.1 months). The OS curve is shown in Figure 1A: 1-, 2-, and 5-year survival rates were 85.0%, 70.0%, and 34.2%, respectively. The median PFS time was 20.0 months (95% CI, 10.9–23.0 months; Figure 1B). The recurrence sites are listed in Table 1. Invasion and/or metastases in the adjacent tissues, such as the pleura, lungs, and pericardium, were observed in thymoma patients. Distant metastases, including in the brain and liver, were common in the thymic carcinoma patients.
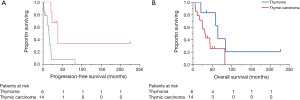
Patients with thymic carcinoma experienced shorter PFS and poorer prognoses compared to the patients with thymoma, although the difference in OS was not statistically significant (median PFS: 15.1 vs. 38.2 months, respectively, P=0.006; median OS: 31.4 and 64.1 months, respectively, P=0.059, 5-year survival: 25.7% and 62.5%; Figure 2A,B).
In three patients, the disease was at stage III; two of these patients had thymic carcinoma. The OS of those with stage III disease ranged from 6.2 to 50.0 months. Patients with stage IVA disease had a median OS of 34.0 months compared to 38.2 months for patients with stage IVB disease.
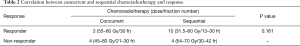
No statistical significant difference was observed between patients who received concurrent and sequential chemoradiotherapy either in survival (median OS: 48.5 vs. 38.2 months, respectively, P=0.83) or response (Table 2). In patients with thymic carcinoma, the median OS for those treated concurrently seemed to be more than 10 months longer than for those treated sequentially (43.5 and 30.4 months, respectively, P=0.81). Platinum-containing chemotherapy did not improve survival compared to other regimens (median OS: 43.5 vs. 53.8 months, respectively, P=0.25).
Full table
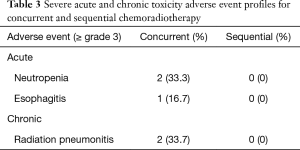
Toxicity profiles are presented in Table 3. All of the adverse events above grade 3 were observed in the patients treated with concurrent therapy (neutropenia, esophagitis, and radiation pneumonia), whereas no severe toxicity was associated with the sequential therapy.
Full table
Discussion
This retrospective analysis involved 20 patients treated with radiotherapy with chemotherapy for unresectable locally advanced thymoma or thymic carcinoma. The chemoradiotherapy-treated patients with thymic carcinoma had a poorer prognosis than those with thymoma.
Almost all the patients with thymic carcinoma were at Masaoka-Koga stage IVA and IVB and survival was similar to that reported in a previous study (14). Past phase II trials in which platinum-containing regimens were administered to patients with stage IV diseases showed that 40–60% of those with thymoma responded compared with only 20–40% of those with thymic carcinoma; the PFSs was reported to be 9–26 and 5–7 months, respectively (15-19). In comparison, the present study showed that 67% of thymoma and 57% of thymic carcinoma patients responded to chemoradiotherapy, and that PFS was 38.2 and 15.1 months, respectively. However, we excluded cases of recurrence after curative-intent surgery, and included patients at lower stages than those in the previous trials. These differences may have resulted in the favorable outcomes of the present study; alternatively, adding radiotherapy to chemotherapy may have achieved better control of the tumors. The 5-year survival rate in our study was 63% for thymoma and 26% in thymic carcinoma, which were similar to those in a previous report (15-19). This may imply that the major cause of death in TETs is not the tumor itself; and it may also indicate that the toxicity of the combination of radiation and chemotherapy could have resulted in deterioration of survival.
Cytotoxic reagents are known to be radiation sensitizers, so a combination of chemotherapy and radiotherapy would be expected to control thymoma and thymic carcinoma (20-22). Given the mechanism of the sensitization, concurrent chemoradiotherapy is recommended for thymic carcinoma (23); however, as yet there has been no study comparing concurrent and sequential chemoradiotherapy in TETs. In the present retrospective study, there was an approximately 10-month difference between concurrent and sequential strategies in both response and survival, although the difference was not statistically significant. This implies that the benefits of radiotherapy could be maximized it applied concurrently with chemotherapy. However, severe adverse events occurred only in the patients who received concurrent therapy, so this indication should be discussed carefully.
Platinum-containing regimens were administered to 80% of patients (n=16) in the present study; however, no significant difference in survival was observed when compared with other regimens without platinum. This may be stem from a synergistic effect of non-platinum cytotoxic drugs, or it may be a result of the size of our study. In our clinical experience, we have assumed that a platinum-doublet regimen such as cisplatin or carboplatin with etoposide, docetaxel, or paclitaxel is the best choice as a combination treatment with radiotherapy based on lung cancer treatment. A recent study on concurrent chemoradiotherapy in non-small cell lung cancer showed the effectiveness of administering S-1 with cisplatin (24) as one of the key drugs for recurrent thymic carcinoma. Furthermore, we have previously reported 85% tumor control in recurrent thymic carcinoma patients treated with S-1 (25). Thus, a regimen containing oral S-1 could be one of the potent chemoradiotherapy regimens for TETs.
Three patients with TETs at Masaoka-Koga Stage III (indicating that the tumors had invaded adjacent organs without distant metastases) were treated with chemoradiotherapy. A previous phase II trial which administrated a cisplatin-containing regimen and a radiotherapy dose of 54 Gy achieved a 5-year survival of 52% in patients with Stage III thymoma (16). None of the patients in the present study survived more than 5 years, perhaps because two of them had thymic carcinoma. The present results indicate that chemoradiotherapy may be effective in controlling thymoma locally, but that the effectiveness deteriorates when the therapy is applied to thymic carcinoma.
In a previous report, the recommended radiation dose for chemoradiotherapy was 60–66 Gy (26). The median dose in our study was 54.5 Gy, but the disease was controlled by the first-line therapy in 85% of the patients. A previous study implied that radiation played an important role in the local control of TETs (27). We have previously reported a case with oligometastasis from thymic carcinoma which was reduced by palliative-intent radiotherapy, with the patient achieving more than 10-year survival after the treatment (28). These results show that TETs may be controlled well by lower doses of radiation, especially in combination with chemotherapy.
ITMIG recommends surgical resection for noninvasive or operable invasive TETs, and that patients with locally advanced disease should receive adjuvant radiotherapy. Radiotherapy is proposed as a standard therapy for unresectable diseases because of the involved organs, but chemoradiotherapy is not mentioned (29). Randomized controlled trials that compare surgery and less invasive therapy are warranted; however, the rarity of the disease makes this difficult. Our previous retrospective study showed that thymic carcinoma patients at Masaoka-Koga stage IVB who underwent surgical interventions had a better prognosis than those who did not receive surgery at the same disease stage (30), although selection bias could not be completely ruled out. The resection of a locally advanced thymic tumor can injure adjacent mediastinal organs, such as causing recurrent nerve damage which leads to a deterioration in the patient’s quality of life. Thus, chemoradiotherapy can be one of the options for patient who are not able or willing to undergo a surgical procedure. However, the effect of combining radiation with chemotherapy is unclear and so careful consideration should be given to the indication.
Our study had some limitations. First, it was a single-institutional retrospective study. Second, as we mentioned earlier, the number of enrolled patients with thymoma or thymic carcinoma was small as these are rare diseases. The effectiveness of chemotherapy for survival was not evident because it is unrealistic to compare a randomized controlled trial with the best supportive care given the nature of rare cancers.
Conclusions
We showed in this study the clinical outcomes of chemoradiotherapy for unresectable TETs. Our results may indicate that the prognosis can be improved by less invasive treatment the patients who cannot receive surgical intervention. Further case accumulation is needed to establish the preferred chemotherapy regimen and the ideal dose of radiation for these cases.
Acknowledgements
The authors thank Makoto Saito, the Senior Biostatistician at the Office for Clinical Research Support in Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, for statistical advice.
Footnote
Conflicts of Interest: Y Hosomi has received speaker fees as honoraria from Eli Lilly Japan K.K., Chugai Pharmaceutical Co., AstraZeneca K.K., Taiho Pharmaceutical Co., and Ono Pharmaceutical Co. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the hospital’s institutional ethics board of Tokyo Metropolitan Komagome Hospital Review Board (No. 1872).
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Engel P, Marx A, Muller-Hermelink HK. Thymic tumours in Denmark. A retrospective study of 213 cases from 1970-1993. Pathol Res Pract 1999;195:565-70. [Crossref] [PubMed]
- Ettinger DS, Riely GJ, Akerley W, et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2013;11:562-76. [Crossref] [PubMed]
- Tseng YH, Lin YH, Tseng YC, et al. Adjuvant Therapy for Thymic Carcinoma – A Decade of Experience in a Taiwan National Teaching Hospital. PLoS One 2016;11:e0146609. [Crossref] [PubMed]
- Rea F, Marulli G, Di Chiara F, et al. Multidisciplinary approach for advanced stage thymic tumors: Long-term outcome. Lung Cancer 2011;72:68-72. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: A phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-46.e1. [Crossref] [PubMed]
- Park S, Ahn MJ, Ahn JS, et al. A Prospective Phase II Trial of Induction Chemotherapy with Docetaxel/Cisplatin for Masaoka Stage III/IV thymic Epithelial Tumors. J Thorac Oncol 2013;8:959-66. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction Chemoradiotherapy Followed by Resection for Locally Advanced Masaoka Stage III and IVA Thymic Tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Travis W, Brambilla W, Müller-Hermelink H, et al. Pathology and genetics of tumors of the lung, pleura, thymus and heart. World Health Organization classification of tumors. Lyon: IARC Press, 2004.
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- ITMIG. Definitions and Policies. J Thorac Oncol 2011;6:S1689-90. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Suster S, Rosai J. Histology of the Normal Thymus. Am J Surg Pathol 1990;14:284-303. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase-II trial of dose-dense chemotherapy in patients with disseminated thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9605). Br J Cancer 2009;101:1549-54. [Crossref] [PubMed]
- Loehrer PJ, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. [Crossref] [PubMed]
- Giaccone G, Ardizzoni A, Kirkpatrick A, et al. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma. A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1996;14:814-20. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II Study of Carboplatin and Paclitaxel in Advanced Thymoma and Thymic Carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality Treatment of Thymoma: A Prospective Study. Ann Thorac Surg 1997;64:1585-91; discussion 1591-2. [Crossref] [PubMed]
- Lucchi M, Mussi A, Basolo F, et al. The multimodality treatment of thymic carcinoma. Eur J Cardiothorac Surg 2001;19:566-9. [Crossref] [PubMed]
- Tamiya A, Matsumura A, Tsuji T, et al. A Pilot Study of Cisplatin and Etoposide With and Without Radiotherapy for Advanced Malignant Thymoma. Anticancer Res 2014;34:2023-7. [PubMed]
- Spaggiari L, Casiraghi M, Guarize J. Multidisciplinary treatment of malignant thymoma. Curr Opin Oncol 2012;24:117-22. [Crossref] [PubMed]
- Ohyanagi F, Yamamoto N, Horiike A, et al. Phase II trial of S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Br J Cancer 2009;101:225-31. [Crossref] [PubMed]
- Okuma Y, Hosomi Y, Miyamoto S, et al. Correlation between S-1 treatment outcome and expression of biomarkers for refractory thymic carcinoma. BMC Cancer 2016;16:156. [Crossref] [PubMed]
- Komaki R, Gomez DR. Radiotherapy for Thymic Carcinoma: Adjuvant, Inductive, and Definitive. Front Oncol 2014;3:330. [PubMed]
- Nonaka T, Tamaki Y, Higuchi K, et al. The Role of Radiotherapy for Thymic Carcinoma. Jpn J Clin Oncol 2004;34:722-6. [Crossref] [PubMed]
- Kashima J, Horio H, Okuma Y, et al. Osseous oligometastases from thymic carcinoma: a case report suggesting the effectiveness of palliative-intent radiotherapy treatment. Onco Targets Ther 2016;9:1029-32. [PubMed]
- International Thymic Malignancy Interest Group. Available online: https://wwwitmigorg/, accessed 5 November 2016.
- Okuma Y, Horio H, Hosomi Y, et al. The potency of curative-intent treatment for advanced thymic carcinoma. Lung Cancer 2014;84:175-81. [Crossref] [PubMed]


