|
Results
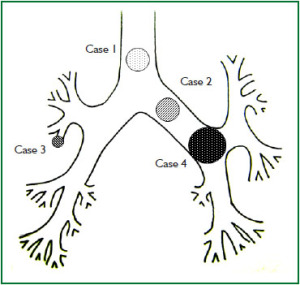
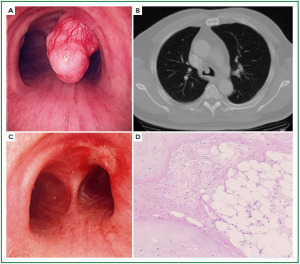
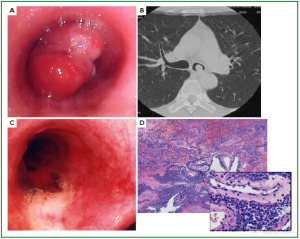
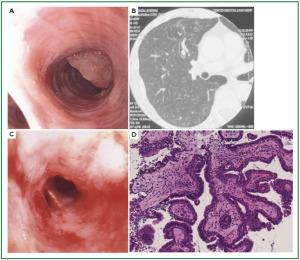
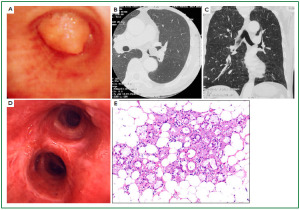
Case 1: Tracheal hamartoma ( Fig 2) A 58-year-old man was admitted to a local hospital with a diagnosis of pneumonia. Chest computed tomography (CT) revealed an intratracheal mass. Chest CT and BF findings showed a pedunculated polypoid tumor with a smooth surface but irregular shape near the right wall of the carina. The tracheal tumor was diagnosed as tracheal hamartoma by biopsy. Because of its morphology and size, we removed the tumor by first strangulating and resecting with an HRF snare, then debulking the residual tumor with an Nd-YAG laser. Finally we used APC to coagulate the base of the lesion to prevent tumor recurrence. Our experience has shown that, to avoid perforation of the airway wall, APC is safer than Nd-YAG laser irradiation to ablate the tumor bed after resection. The results of pathological diagnosis were hamartoma ( 12, 13), as shown in Fig 2. There has been no recurrence during the 112 months of outpatient follow-up. Case 2: Bronchial glomus tumor in the left main bronchus ( Fig 3) A tumor was detected on the chest CT of a 39-year-old man with cough and bloody sputum, and the BF findings revealed a smooth lesion occupying more than 90% of the left main bronchial lumen. The left main bronchus tumor appeared to be a pedunculated polypoid lesion with a smooth, red mucosal surface. Transbronchial fine needle aspiration cytology (TBAC) did not yield a definitive histological diagnosis of the tumor. We therefore performed an exploratory procedure under general anesthesia with spontaneous respiration using a rigid scope. When we resected the distal root of the tumor with electrocautery, there was marked bleeding (@ 200ml) from the stump. Hemostasis was achieved by a combination of YAG laser and MW coagulation. The cytological findings of preoperative TBAC showed close clusters of round or polygonal small cells. On intraoperative frozen section, atypical small round tumor cells showed hyperplasia with amorphia. It was difficult to establish a definitive diagnosis, because of its featureless structure, nevertheless carcinoid or adenoid cystic carcinoma were nominated as likely differential diagnoses. Moreover, no other distinguishing structures could be seen pathologically. However, cells with small circular nuclei, slightly acidophilic bright cytoplasm, as well as many capillaries were recognized in the tumor tissue. The tumor cells were hyperplastic when they surrounded blood vessels, and neither ductal structures nor organic patterns could be seen in the tissue. No evidence of malignancy such as strong dyskaryosis or prominent nuclear division could be seen. The results of immunohistochemical staining for muscle actin (HHF35) and α-smooth muscle actin (SMA) were positive, but negative for cytokeratin AE1/AE3 and CAM5.2, epithelial membrane antigens (EMA), chromogranin, synaptophisin and neural cell adhesion molecule (NCAM; CD56). Since some benign tumors recur after treatment, it is essential to fully grasp the histological character and establish a pathological diagnosis. Finally, the tumor was diagnosed as a bronchial glomus tumor ( 14- 16). No recurrence of the tumor has been seen during the 86 months of follow-up. Case 3: Papilloma in the right subsegmental bronchus ( Fig 4) We recognized a small polypoid mass, protruding from the left wall of the right B3b in a 52-year-old woman, causing obstructive pneumonitis of the right upper lob on chest CT. BF revealed a tumor with an irregular smooth surface. Biopsy yielded a pathological diagnosis of papilloma ( 17- 19). Polypectomy was performed under local anesthesia. Since initial use of the HRF snare did not remove the entire lesion, the residual lesion was removed by forceps because of the proximity of a blood vessel, via the BF. We have been following up this recurrence-free patient for 87 months. Case 4: Lipoma in the left main bronchus ( Fig 5) The chest CT of a 58-year-old man showed a round mass 2 cm in diameter in the left main bronchus with a density level similar to fat tissue. BF findings showed a round polypoid tumor with a distal border between the tumor and normal tissue. The pedunculated tumor in the left main bronchus moved on respiration. Confirmation of the tumor by BF and biopsy revealed lipoma even on the distal side. Tumorectomy was performed with an HRF snare via BF under local anesthesia. The final pathological result of this tumor was sialolipoma, a benign lipomatous tumor occurring in salivary glands ( 10, 20). Pathological findings showed a well-circumscribed mass composed of glandular tissue and mature adipose elements. The glandular components resembled normal salivary gland tissues. There has been no recurrence at 51 months. |
Discussion
We set out to provide a concise overview of various benign neoplasms of the airway in a variety of possible locations and to describe the available range of approaches to diagnosis and treatment. The incidence of benign tumors in the central airway is low, and there are extremely few reports on series of such benign tumors. In the series reported by Grillo et al. ( 21), most of their 198 primary tracheal tumors were malignant, and the frequency of benign tumors was 10.6%, i.e. 1.9% of all lung tumors ( 2). Most information on benign bronchial tumors is contained in individual case reports, which does not promote understanding of this entity. As a large referral center for pulmonary diseases, we have had the opportunity to encounter a relatively wide range of benign bronchial lesions, and here we present retrospectively selected cases that contribute to evaluation of representative management and strategies of interventional bronchology for these entities and the merits and demerits of each technique ( Table 2). Strategy and examination
It is relatively rare for bronchial tumors to show an abnormal shadow on chest X-ray films. In some such cases, we employed conservative treatment under a diagnosis of bronchial asthma or bronchitis even though they had initially actually had had a bronchial tumor. Concerning clinical symptoms, dyspnea can show rapid exacerbation in benign lesion cases when tumor causes stenosis of the tracheal lumen of over 50% ( 22, 23). Therefore these tumors, particularly tracheal tumors, can require much time to establish a definitive diagnosis. In this regard, patients showing respiratory symptoms continuously, unaffected by seasonal change, asthmatic patients who are minimally responsive to drugs such as bronchodilators, and patients with recurrent pneumonia must be distinguished from patients with bronchial tumors ( 24). It is sometimes necessary to perform chest CT, magnetic resonance image (MRI) and BF for patients suspected to have bronchial tumors. The thin-slice CT images and 3-dimensional CT (3D CT) which can display minute details are able to not only suggest tumor characteristics, but also determine whether the pedunculated mass has a narrow stalk, or is broad-based, extending across multiple cartilaginous rings, and demonstrate extramural tumor invasion, or the presence of distal lesions. Pretherapeutically, it is particularly necessary to accurately grasp the extramural vascular anatomy and confir the tumor position, size, surface appearance and degree of tumor mobility. BF (under local anesthesia) or rigid scope (under general anesthesia)?
Therapeutic strategy selection is also very important. Selection of BF with local anesthesia or a rigid scope with general anesthesia depends on conditions such as the location, size, shape, histologic type of tumor and respiratory status. However, general condition, age, cardiorespiratory state and coexistent disease are also important factors regarding choice of treatment. Usually, we use a BF under local anesthesia with the patient in an awake state if there are no problems in the overall status. Reasons for this approach include consideration of the physical burden and risk of complications for patients undergoing general anesthesia, and economic aspects such as length of hospital stay. On the other hand, if patients have a low pulmonary function, are elderly, or have a history of heart cardiac disease, treatment under general anesthesia can be desirable from the viewpoint of safety ( 25, 26). If the tumor resection is supervised by an anesthesiologist, emergency events can be handled with maximum efficiency. General anesthesia should be selected over awake local anesthesia if tumorectomy is anticipated to require a long time (more than 30 minutes) or if the anatomical location may be problematic, such as in the right upper lobe bronchus. Although in most cases, we use the rigid scope to deal with massive hemorrhage after resection of an inflammatory lesion or tuberculous mass; in cases with perforation of the bronchial wall with high power laser irradiation; voluminous sputum from the peripheral side after opening the site of tumor occlusion; or tumors too large to be able to be removed with conventional forceps ( 24, 26). Use of the rigid scope is also recommended if large blood vessels are recognized near the tumor. Intervention or surgical treatment?
While endoscopic treatment is frequently the best choice, careful consideration about the choice and application of therapeutic implements is essential before treatment. When the possibility of malignancy cannot be denied, or when there are indications for surgical resection because of peripheral pulmonary organizing pneumonia or bronchiectasis due to repeated infection, surgical resection of the lung together with the bronchial tumor, is frequently performed ( 2). The following conditions are considered as indications for surgical resection, rather than endoscopic treatment: when malignancy cannot be denied histologically, cases accompanied with organizing pneumonia or remarkable bronchodilation of the peripheral side beyond the tumor ( 2), tumors with extensive infiltration of the bronchial wall across more than 3 bronchial cartilage rings or appearing to invade beyond the tracheal wall ( 2). Furthermore, endoscopic therapy is not indicated in cases in which it is not possible to confirm the status of the peripheral bronchus beyond the point of tumor obstruction, or cases in which fatal hemorrhage might be caused by bronchial wall perforation. In such cases, the possibility of malignant tumor cannot be denied preoperatively and therefore surgical resection is indicated. When carcinoma remains at the resected stump, sleeve lobectomy and cylindrical tracheal resection with end-to-end anastomosis should be considered. These therapeutic methods can also help maintain respiratory function. However, if surgery or combined surgery and other methods are contraindicated due to low pulmonary function or poor general status, the following endoscopic techniques may be used. High power laser (wavelength 1020~1064 nm): Diode / Nd -YAG laser
High power laser irradiation is often considered to be the first treatment of choice for emergency airway maintenance. The effective depth of laser power is several mm to several cm, depending on the laser power output. If the length of tracheal cartilage destruction is 4 cm or less ( 27), tangentially applied high power laser up to 40 W is indicated. Care is needed to avoid too much power per shot, and for irradiation of surrounding normal tissue the setting should be lower than 40 W. Dividing laser irradiation into several shots, while continuously confirming the results is the safest method. Careful examination is necessary to prevent bronchial wall perforation and massive bleeding from surrounding blood vessels. Massive bleeding during treatment may be controlled by epinephrine saline spray in the airway or compression by the cuff of the tracheal tube. However, in the few cases of pulmonary artery bleeding from the carina, main bronchus or truncus intermedius, the hemorrhage is often massive, control is difficult, and the results can be catastrophic, therefore it is essential to perform contralateral intubation to maintain the airway, and high power laser cauterization while periodically checking the condition of the tumor. Inhalation of cauterization fumes can aggravate respiratory status through airway damage. We therefore have to remove tissue cauterization fumes in synchrony with patient exhalation, thereby minimizing absorption of fumes.
High power laser therapy usually induces reactive hyperplasia of granulation immediately, even if there is temporary regression of inflammatory granular tissue, which is the reason why it is considered unsuitable for tuberculosis lesions. Cauterization of excessively large areas of tumor at a single time can induce temporary edema and exacerbate airway stenosis. Several pulmonologists reported ( 23, 27, 28) that laser therapy using high concentrations of oxygen of more than 40% fraction inspired oxygen concentration (FiO 2) might cause airway fire. Operators must continuously monitor oxygen concentration in the airway, and refrain from high-power laser therapy in cases requiring persistent high-concentration oxygen administration. MW solidification (3-5)
Tissue irradiated with MW solidifies, and compared with high power laser, MW solidification has the advantages of less bleeding and minimal smoke inhalation. MW solidification has a low risk of bronchial wall bleeding or perforation. This method also permits the use of high concentration oxygen inhalation. Although the cauterization power of MW solidification is weak and therefore safer (the power of normal MW for tissue solidification generally needs 20 W output), it is suitable for a wide range of tissue solidification procedures. High-power laser is monodirectional and can be irradiated only in a straight line directly toward the target. However, MW has the advantage of irradiation not only in a straight line but also from any approach angle, even tangential to the target. If the tumor is located at a bifurcation with an acute angle such as in the bilateral upper lobe bronchi, it would probably be best to perform MW initially.
APC solidification (6-8)
APC involves solidification with a non-contact type monopolar electrode. The high-frequency current reaches the target tissue along with the flow of ionization argon. In argon plasma, the electric current flows toward tissue with least resistance and solidifies tissue surface equally so that it spreads to the non-solidified part and bleeding parts of tissue. The depth of solidified tissue is controlled automatically. Only the tissue surface shows APC solidification effect and its cauterization range is limited. Therefore while this therapy has a low risk of perforation of the bronchial wall, it is not optimal for large pedunculated tumor resection.
HRF snare (9, 10)
As its name implies, the HRF snare enables delivery of alternating current into tissue at a high frequency (105-107 Hz). The strong heating action of this device enables both tissue solidification and tumor resection. This can be effective in initial treatment, especially for polypoid-type tumors occluding central airways, particularly for poorly vascularized pedunculated polyps. While the HRF snare has a low risk of complications due to fumes or aggravation of respiratory status during cauterization and burn edema of the airway after treatment, this technique can be difficult for wide-based tumors. One merit of the HRF snare is that it can resect a tumor mass in a short time, and with little histological change of the resected specimen, unlike when laser or MW irradiation are used.
Dehydrated ethanol injection therapy (11)
Dehydrated ethanol injection therapy can be effective in cases of possible hemorrhage or at high risk for bleeding. Usually the injection dosage is about 0.2 - 0.3 ml dehydrated ethanol per site until the surrounding mucosa turns white. This method is particularly useful preventing hemorrhage before cauterization of the base of a tumor. This treatment is not only effective with tumor tissue but also with inflammatory granulation tissue.
Risk management
When performing interventional bronchology, many considerations must be kept in mind; during treatment patients must be attached to a pulse oxygen monitor and an electrocardiogram monitor to grasp the cardiopulmonary status, and preparations for emergency airway maintenance are necessary at all times. If interventional techniques under local anesthesia are insufficient, it may be necessary to convert to rigid endoscope under general anesthesia.
The most important point in airway cauterization is oxygen concentration control. In cauterizing during tracheal intubation, we must maintain oxygen concentration below 30%. Post treatment management, such as steroid administration, is essential for the prevention of airway mucosal edema. Removal of tumors greater than 2 cm in diameter may require tracheostomy ( 29). Furthermore, since some tumors show recurrence and malignant transformation even after resection, careful periodical BF observation of the resection stump is necessary. Limitations
The main limitation of this study is the small number of cases. However we believe that this is a good reference manuscript for the description and characteristics of the various therapeutic options available with both rigid and flexible broncoscopes in the treatment of benign airway tumors.
|
|
Conclusions
Interventional bronchology techniques for benign bronchial tumor are simple and usually safe, and can be immediately effective, even in patients in whom surgery is contraindicated due to insufficient cardiopulmonary reserve or poor general status.
|
|
Acknowledgements
The authors are grateful to Prof. J. Patrick Barron, Department of the International Medical Communications of Tokyo Medical University for reviewing the English manuscript. All of the authors participated in this study and agreed to the content of this paper. None of them have any financial or other relations that could lead to a conflict of interest.
|
|
References
- Godard P, Draussin M, Lopez FM, Romieu M, Miro L, Michel FB, et al. The use of a laser beam in bronchology. Resection of 2 tracheo-bronchial tumours (author's transl). Poumon Coeur 1979;35:147-50.[LinkOut]
- Shah H, Garbe L, Nussbaum E, Dumon JF, Chiodera PL, Cavaliere S. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest 1995;107:1744-51.[LinkOut]
- Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42.
[LinkOut]
- Suito T, Tani G, Seki T, Kikuchi K. Bronchoscopic Microwave Tissue Coagulation for Respiratory Tract Obstruction. J Jpn Soc Resp Bronchology 1989;11:88-92.[LinkOut]
- Seto T, Semba H, Fukai Y, Uchimura A, Kurano R. Coagulation of Intratracheal and Bronchial Tumors by Transbronchoscopic Microwave Electrodes. J Jpn Soc Resp Bronchology 1996;18:430-6.[LinkOut]
- Reichle G, Freitag L, Kullmann HJ, Prenzel R, Macha HN, Farin G. Argon plasma coagulation in bronchology: a new method--alternative or complementary? Pneumologie 2000;54:508-16.[LinkOut]
- Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2:42-6.
[LinkOut]
- Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7.
[LinkOut]
- Hintze RE, Adler A, Veltzke W. Endoscopic resection of large colorectal adenomas: a combination of snare and laser ablation. Endoscopy 1995;27:665-70.
[LinkOut]
- Muraoka M, Oka T, Akamine S, Nagayasu T, Iseki M, Suyama N, et al. Endobronchial lipoma: review of 64 cases reported in Japan. Chest 2003;123:293-6.
[LinkOut]
- Fujisawa T, Hongo H, Yamaguchi Y, Shiba M, Kadoyama C, Kawano Y, et al. Intratumoral ethanol injection for malignant tracheobronchial lesions: a new bronchofiberscopic procedure. Endoscopy 1986;18:188-91.
[LinkOut]
- Taştepe AI, Kuzucu A, Demircan S, Liman ST, Demirağ F. Surgical treatment of tracheal hamartoma. Scand Cardiovasc J 1998;32:239-41.
[LinkOut]
- David O, Beasley MB, Minardi AJ Jr, Malek F, Kovitz KL. Management of endobronchial hamartoma. J La State Med Soc 2003;155:110-2.
[LinkOut]
- Lange TH, Magee MJ, Boley TM, Bell SW, Hazelrigg SR. Tracheobronchial glomus tumor. Ann Thorac Surg 2000;70:292-5.
[LinkOut]
- Oizumi S, Kon Y, Ishida T, Yamazaki K, Itoh T, Ogura S, et al. A rare case of bronchial glomus tumor. Respiration 2001;68:95-8.
[LinkOut]
- Vailati P, Bigliazzi C, Casoni G, Gurioli C, Saragoni L, Poletti V. Endoscopic removal of a right main bronchus glomus tumor. Monaldi Arch Chest Dis 2004;61:117-9.[LinkOut]
- Fechner RE, Fitz-Hugh GS. Invasive tracheal papillomatosis. Am J Surg Pathol 1980;4:79-86.[LinkOut]
- Shapshay SM, Simpson GT 2nd. Lasers in bronchology. Otolaryngol Clin North Am 1983;16:879-86.
[LinkOut]
- Spencer H, Dail DH, Arneaud J. Non-invasive bronchial epithelial papillary tumors. Cancer 1980;45:1486-97.[LinkOut]
- Spinelli P, Pizzetti P, Lo Gullo C, Rocca F, Gobbi A, Ravasi G. Resection of obstructive bronchial fibrolipoma through the flexible fiberoptic bronchoscope. Endoscopy 1982;14:61-3.[LinkOut]
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77.[LinkOut]
- Beamis JF Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med 2005;11:292-5.[LinkOut]
- Sohrab S, Mathur PN. Management of central airway obstruction. Clin Lung Cancer 2007;8:305-12.[LinkOut]
- Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest 2007;131:261-74.[LinkOut]
- Sutedja G, van Kralingen K, Schramel FM, Postmus PE. Fibreoptic bronchoscopic electrosurgery under local anaesthesia for rapid palliation in patients with central airway malignancies: a preliminary report. Thorax 1994;49:1243-6.[LinkOut]
- Sutedja G, Bolliger CT. Endobronchial electrocautery and argon plasma coagulation. Interventional bronchoscopy. Prog Resp Respir. Basel Karger 2000;30:120-32.[LinkOut]
- Kvale PA, Eichenhorn MS, Radke JR, Miks V. YAG laser photoresection of lesions obstructing the central airways. Chest 1985;87:283-8.[LinkOut]
- Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer. Laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med 2002;23:241-56.[LinkOut]
- Kamiyama K, Yamamoto T, Morita R, Suzuki Y, Akaoqi E, Mitsui K. A Case of Tracheal Tumor Resected by Bronchofiberscopic Polypectomy. J Jpn Soc Bronchology 1989;11:576-9.[LinkOut]
Cite this article as: Kajiwara N, Kakihana M, Usuda J, Ohira T, Kawate N, Ikeda N. Interventional management for benign airway tumors in relation to location, size, character and morphology. J Thorac Dis 2011;3(4):221-230. doi: 10.3978/j.issn.2072-1439.2011.04.06
|
|