Endoscopic diagnosis and management of pleural effusion in malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive tumor that has become a very important issue over recent years (1,2). Asbestos exposure is the main factor involved in pathogenesis. Its wide use in construction explains the rise in incidence of MPM since the 1960s. It is estimated that the incidence of MPM will increase with a peak in the next 5 years (1,3).
The diagnosis of MPM is difficult because of many reasons, including clinical, radiological, laboratory and pathological factors (4-6).
Clinical manifestations of MPM are usually nonspecific and insidious, with mild breathlessness, nonspecific thoracic pain, weight loss, fatigue. The history of a previous asbestos exposure heightens the clinical suspicion, but is sometimes difficult to assess (1).
At present some blood biomarkers, such as mesothelin-related peptide and osteopontin, are available, but without sufficient diagnostic reliability (6,7).
Chest X-ray (CXR) usually shows nonspecific findings, such a unilateral pleural effusion in the early stages, or pleural thickening in later stages (4,6,7).
Chest Computed Tomography (CT) scan is important in the initial assessment, potentially showing nodular or diffuse pleural thickening, which can be suggestive of the disease (1); the latest CT technology (>32 detectors rows) with multiplanar reconstructions allows a good assessment of chest wall, pericardium and diaphragm invasion. However CT alone is unsuitable for a definite diagnosis (1,7).
Chest magnetic resonance is not deemed a relevant diagnostic tool, and may be useful in selected cases (1,4,6,7).
Positron emission tomography (PET)/CT scanning may be helpful in determining the local extent of MPM, but without sufficient accuracy compared to the endoscopic evaluation (8,9).
Chest ultrasound (US) is very useful in assessing pleural effusions and can identify thickenings or nodules, although without specificity for MPM (10,11).
Pleural fluid cytology is a diagnostic tool for MPM only for experienced cytopathologists, and when no tissue is achievable (7); otherwise it can only lead to a suspicion of the disease. For a definitive diagnosis it is necessary to demonstrate the presence and the histological architecture of neoplastic mesothelial cells in the pleura and in the underlying layers, with immunohistochemical characterization (1,4-7).
There is a definite consensus on medical thoracoscopy (MT) as the main diagnostic tool for MPM (1,12). It allows a complete macroscopic evaluation of the pleural cavity with staging implications, and the execution of multiple biopsies. Besides, it is possible to get in a single procedure the diagnosis and the control of the pleural effusion, which is one of the main clinical problems in the evolution of MPM, causing worsening dyspnea and discomfort to the patient. In order to prevent its recurrence, performing a pleurodesis procedure during MT is therefore essential (12).
Medical thoracoscopy
MT also named pleuroscopy or local anesthesia thoracoscopy is a minimally invasive technique that allows detailed examination of the pleural cavity and targeted biopsies of the parietal pleura (12). It actually represents the method of choice to investigate unexplained exudative pleural effusions, after an inconclusive pleural fluid analysis (13).
MT is different from surgical thoracoscopy, since it is performed by pulmonologists using moderate sedation in spontaneously breathing patients, with no need of intubation or mechanical ventilation.
Furthermore, when needed MT allows the treatment of recurrent pleural effusion in the same setting, with pleurodesis or the insertion of an indwelling pleural catheter (12).
In recent years the use of bedside US has become very popular among pulmonologists, and at present should be considered part of the clinical examination before thoracoscopy (14). It provides very useful information about the amount of pleural fluid, its characteristics, the presence and the site of loculations not easily predictable on CT scans, the potential presence of large adhesions, and the localization of some parietal neoplastic lesions. Therefore it facilitates the identification of the best trocar entry site (15-18).
Moreover, it permits with high sensitivity the assessment of the so-called pleural “sliding sign”, which represents the physiological movement of the lung on the parietal pleural surface during the respiratory activity (10,15). When present, this sign shows the absence of adhesions and the possibility of a pneumothorax to be induced and the thoracoscopy to be performed, even in the complete absence of pleural effusion. In these cases an iatrogenic pneumothorax can be created using the Boutin needle (19), or with the direct careful insertion of the trocar (20).
The usual equipment includes a trocar and a rigid 7 mm endoscope, direct (0°) and lateral (50°) optics, connected to a cold-light source and to a video camera system, and optical forceps for the biopsies of the parietal pleura.
The procedure is usually performed under local anesthesia and moderate sedation, generally without the need of intubation, with the patient continuously monitored with pulse oximetry, blood pressure measurements and electrocardiogram tracing. In an ideal setting the presence of an anesthesiologist is required, with resuscitation equipment readily available. A surgical backup must be accessible in case of intraoperative complications needing a conversion to thoracotomy (12).
The patient is typically positioned in the lateral decubitus position with the affected side upward. The main operator faces the patient, allowing an optimal approach to the parietal pleura.
A small skin incision followed by blunt dissection is performed to introduce the trocar in the pleural space. The thoracoscope can be inserted and the pleural fluid evacuated, allowing the operator to explore the pleural cavity through the optics, cutting or breaking subtle adhesions, collecting fibrin samples and performing pleural biopsies. At the end of these interventions, a pleurodesis with talc poudrage can be done based on clinical judgment, and a chest tube connected to a water seal device must be inserted. As an alternative, a tunneled indwelling pleural catheter may be placed (12).
Thoracoscopic findings
The first priority during thoracoscopy is to check the presence of large adhesions, because they can prevent the adequate exploration of the pleural cavity, potentially missing neoplastic lesions. However, even an incomplete view of the pleural surface sometimes allows the identification of neoplastic pleural areas, which must be searched especially in the lower parts of the cavity and in the costophrenic angles, where the MPM is used to growing at the onset (21,22).
The second priority is a good knowledge of the macroscopic normal appearance of the pleura and of its pathological processes, in order to clearly identify inflammatory or neoplastic lesions, irrespective of their origin (23,24).
The elementary pleural neoplastic lesions are nodules, masses, thickening and malignant pachypleuritis (21,22).
Nodules are lumps between 1 and 10 mm in diameter, either isolated or more frequently numerous and widespread.
Masses are lesions larger than 10 mm in diameter, often confluent.
Thickening is represented by areas of tissue of several millimeters, with irregular surface, ill-defined borders, whitish aspect, poor vascularization, and infiltrative aspect.
Malignant pachypleuritis is a diffuse thickening, usually whitish, involving a large part or the whole pleural cavity.
Along with clinical, CT and US data, these lesions contribute to define three main patterns of the thoracoscopic appearance of MPM: multinodular, pachypleuritis and nonspecific (21,22).
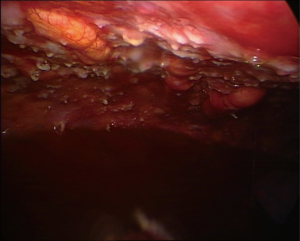
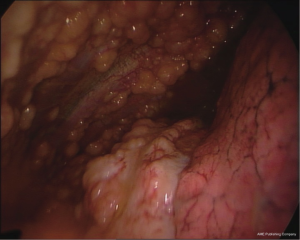
The multinodular pattern (Figure 1) is often predictable on CT scans and on chest US, especially when large nodular structures can be better identified in the presence of pleural fluid; the endoscopic appearance is mostly a diffuse spread of nodular lesions, with a particular predominance in the middle and lower regions of the parietal pleura, especially in the costophrenic angles and on the diaphragm surface, even if all the pleural surface can be affected.
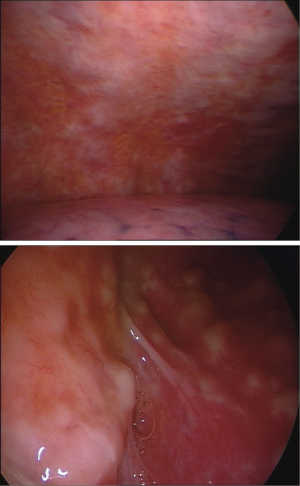
The pachypleuritis pattern (Figure 2) can be predicted on CT scans by the retraction of the hemithorax, on CT and US by the presence of parietal linear thickenings, often in the absence of pleural effusion; the endoscopic appearance shows a hard whitish tissue covering all the parietal pleural surface, often with ribs structures no longer visible. The visceral pleura can be involved in the area of the fissures.
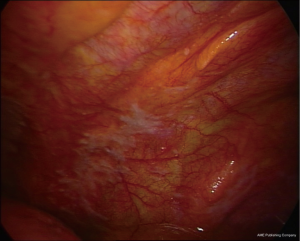
The nonspecific pattern (Figure 3) is associated with the sole pleural effusion on CT, whereas endoscopy shows mostly an inflammatory picture, sometimes with small areas of thickening in the lower regions of the parietal pleura, and areas with granular surface, suggestive of lymphangitis.
When neoplastic lesions are identified during thoracoscopy, it is substantially impossible to determine their nature based on their sole macroscopic appearance, even if there are some clues particularly suggestive of MPM.
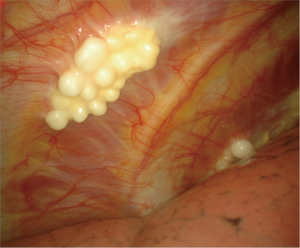
The presence of fibrohyaline plaques (areas of hard whitish fibrous tissue, with well-defined borders, sometimes with pearl-like nodules) (Figure 4) indicates a previous asbestos exposure, and is often associated with MPM (23,24).
Other clues are the diaphragm involvement, the presence of visceral lesions limited to the fissures, the involvement of the adhesions, a rigid and hard parietal pleura, areas of multiple confluent whitish or yellowish translucent nodules with a grape-like aspect (Figure 5), parietal black spots, mixed macroscopic pattern (Figure 6).
Biopsies technique
In suspected cases of MPM, it is important to perform the most extensive exploration of the pleural cavity as possible, looking for neoplastic lesions on the whole diaphragmatic and parietal surface, and, as far as possible, on the visceral pleura and the fissures, for a correct staging.
Once evacuated the pleural fluid, explored the cavity and located the lesions, multiple biopsies are performed using forceps mounted on the direct optics.
In order to get material sufficiently representative of the tumor and to allow a thorough pathological and immunohistochemical characterization, it is essential to perform multiple, deep and large biopsies (1,21,25).
For this purpose “biopsies on biopsies” are performed, digging the tissue in the same point to obtain fat and/or muscle samples to evaluate tumor invasion, and biopsies peeling the pleural surface to get large areas of tissue. It is also recommended to take biopsies of both normal and seemingly abnormal pleura (1).
Taking biopsies during MT is generally safe with some precautions, and only a self-limiting mild bleeding may occur. It is important to avoid lung apices and anterior parietal pleura because of the presence of subclavian and internal mammary arteries, respectively. Another highly vascularized region is the diaphragmatic pleura, whereas the inferior portion of the posterior parietal pleura is considered safe (12).
A major bleeding is a rare complication and can be generally managed by external compression of the intercostal space or internal compression with closed forceps holding folded gauze. Rarely a conversion to thoracotomy with ligation of bleeding vessels is required (12).
MT is a relatively safe procedure, with a mortality rate estimated at 0.34% (26).
Contraindications
The absence of a pleural space such as in the setting of extensive pleural adhesions or previous pleurodesis is the only absolute contraindication. When extensive adherences are present, it may be virtually impossible to perform a MT because they can prevent the lung collapse (12). This can be the indication for alternative procedures, such as a CT-guided or ultrasound-guided transthoracic needle biopsy or a surgical procedure, such as video-assisted thoracoscopic surgery (VATS) or thoracotomy. However the diagnosis of MPM from needle biopsies (e.g., Abrams needle, Cope needle, Tru-cut, etc.) is associated with the same limits as cytology, regarding the scarcity of material representative of the tumor (1).
Other contraindications are represented by critical clinical conditions (e.g., hemodynamic instability, severe hypoxemia, uncorrectable coagulopathy) (12).
Management of pleural effusion
MPM is a highly aggressive tumor and the clinical course is characterized by chronic symptoms such as chest pain and progressive dyspnea, with a significant impact on quality of life (QoL). The main cause of dyspnea is the presence of recurrent pleural effusion, which can often be massive. When patients complain of this symptom, a palliative intervention is required (1,6,7).
The diagnosis of pleural effusion is easily done by CXR or thoracic US. Once the presence of pleural effusion has been assessed, different management approaches can be taken depending on performance status of the patient, the response to systemic therapy and the degree of lung re-expansion following pleural fluid evacuation. Options for management include thoracentesis, pleurodesis or placement of an indwelling pleural catheter (IPC).
Thoracentesis
Thoracentesis consists in pleural fluid aspiration and can provide only a transient relief of symptoms. Repeated procedures may be indicated for patients with very limited survival expectancy and poor performance status. It can be easily performed in an outpatient setting, avoiding hospitalization. It is associated with a high rate of pleural fluid recurrence. On the other hand the risk of complications is very low (13).
Pleurodesis
Pleurodesis aims to obliterate the pleural space by producing extensive adhesions of the visceral and parietal pleura, preventing the recurrence of pleural fluid. This should be directed to those patients who complain of chronic symptoms and have experienced symptomatic improvement after therapeutic thoracentesis.
The procedure can be generally divided into mechanical and chemical approaches. Mechanical pleurodesis encompasses mechanical irritation of the pleura and the removal of parietal pleura and is essentially a surgical procedure. Chemical pleurodesis is performed by introducing sclerosant substances such as talc, bleomycin, povidone iodine or other chemical substances into the pleural space through a catheter or during MT, after the removal of the pleural fluid. Chemical sclerosants cause irritation of the pleural layers and thus diffuse inflammation and subsequent formation of fibrin adhesions. Talc is considered the best sclerosant for pleurodesis regarding the rate of success (13). It can be administered as “talc slurry” (a suspension of sterile graded talc in 0.9% saline) through a chest tube, or as “talc poudrage” (insufflation of sterile graded talc powder during MT or VATS), with substantial equivalence in efficacy (27,28).
The effectiveness of the pleurodesis is strongly dependent on the largeness of the healthy pleural surface, and therefore it is higher especially in the early stages of disease.
Likewise the wider area of apposition between the two serous membranes is present, the more effective the procedure will be.
Thus it is important to achieve an adequate pulmonary re-expansion after fluid removal, prior to performing pleurodesis; trapped lung (due to a thick visceral peel), pleural locations, persistent air leaks or proximal large airways obstruction lead to an incomplete lung re-expansion and thus are considered contraindications to the procedure (13).
Indwelling pleural catheters
IPCs are multi-fenestrated flexible chest drains, tunneled in the subcutaneous tissue in the medial portion, provided with a one-way valve designed to be attached to vacuum drainage bottles. Catheters are generally inserted on an outpatient basis under local anesthesia. Patients are educated to manage the catheter at home and to evacuate the fluid when needed. The aim of this device is to intermittently drain the effusion without a more specific attempt at causing pleurodesis, so even in cases of trapped lung. IPCs are now established as one of the major tools for the management of recurrent pleural effusion (29,30).
Conclusions
MPM is an aggressive tumor related to asbestos exposure characterized by an insidious onset and poor clinic. Supported by radiological tools, a definitive diagnosis can be achieved by MT, a low risk procedure. A thorough chest assessment by US can be now considered essential part of the procedure, allowing the study of the chest wall, the parietal pleura, the effusion, and eventually the identification of the best trocar insertion site. MT permits to obtain large biopsies for a correct pathological diagnosis, contributes to the staging and allows the talc poudrage pleurodesis. The control of recurrent pleural effusion is crucial in the management of these patients, with palliative purpose. Talc pleurodesis is the most popular procedure aimed to control the recurrence of effusion, and in recent years IPCs have become suitable alternatives.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. [Crossref] [PubMed]
- Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93. [Crossref] [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v31-9. [Crossref] [PubMed]
- Rodriguez-Panadero F, Janssen JP, Astoul P. Thoracoscopy: general overview and place in the diagnosis and management of pleural effusion. Eur Respir J 2006;28:409-22. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian consensus conference on malignant pleural mesothelioma: state of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- Pinelli V, Roca E, Lucchini S, et al. Positron Emission Tomography/Computed Tomography for the Pleural Staging of Malignant Pleural Mesothelioma: How Accurate Is It? Respiration 2015;89:558-64. [Crossref] [PubMed]
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis. Acad Radiol 2014;21:11-20. [Crossref] [PubMed]
- Zanforlin A, Giannuzzi R, Nardini S, et al. The role of chest ultrasonography in the management of respiratory diseases: document I. Multidiscip Respir Med 2013;8:54. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii61-76. [Crossref] [PubMed]
- Skalski JH, Astoul PJ, Maldonado F. Medical thoracoscopy. Semin Respir Crit Care Med 2014;35:732-43. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Michaud G, Ernst A. Ultrasound and medical thoracoscopy. In: Bolliger CT, Herth FJF, Mayo PH, et al. Clinical chest ultrasound from the ICU to the bronchoscopy suite. Basel: Karger, 2009:182-8.
- Smargiassi A, Inchingolo R, Soldati G, et al. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med 2013;8:55. [Crossref] [PubMed]
- Wei B, Wang T, Jiang F, et al. Use of transthoracic ultrasound to predict pleural adhesions: a prospective blinded study. Thorac Cardiovasc Surg 2012;60:101-4. [Crossref] [PubMed]
- Sasaki M, Kawabe M, Hirai S, et al. Preoperative detection of pleural adhesions by chest ultrasonography. Ann Thorac Surg 2005;80:439-42. [Crossref] [PubMed]
- Medford AR, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [Crossref] [PubMed]
- Corcoran JP, Psallidas I, Hallifax RJ, et al. Ultrasound-guided pneumothorax induction prior to local anaesthetic thoracoscopy. Thorax 2015;70:906-8. [Crossref] [PubMed]
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [Crossref] [PubMed]
- Boutin C, Rey F. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer 1993;72:389-93. [Crossref] [PubMed]
- Boutin C, Schlesser M, Frenay C, et al. Malignant pleural mesothelioma. Eur Respir J 1998;12:972-81. [Crossref] [PubMed]
- Gevenois PA, de Maertelaer V, Madani A, et al. Asbestosis, pleural plaques and diffuse pleural thickening: three distinct benign responses to asbestos exposure. Eur Respir J 1998;11:1021-7. [Crossref] [PubMed]
- Bianchi C, Brollo A, Ramani L, et al. Pleural plaques as risk indicators for malignant pleural mesothelioma: a necropsy-based study. Am J Ind Med 1997;32:445-9. [Crossref] [PubMed]
- Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J 1998;11:213-21. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Clive AO, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016.CD010529. [PubMed]
- Fortin M, Tremblay A. Pleural controversies: indwelling pleural catheter vs. pleurodesis for malignant pleural effusions. J Thorac Dis 2015;7:1052-7. [PubMed]
- Bhatnagar R, Maskell NA. Indwelling pleural catheters. Respiration 2014;88:74-85. [Crossref] [PubMed]