Tumor-associated macrophages—additional effectors at anti-PD-1/PD-L1 therapy?
Immunotherapies that target programmed cell death protein 1 (PD-1) or one of its ligands, programmed cell death ligand 1 (PD-L1), are a recent breakthrough in treatment of human malignant diseases (1), including non-small cell lung cancers (NSCLCs) (2). Monoclonal antibody drugs that target the interaction between PD-1 and PD-L1 have shown dramatic and/or durable responses in a subset of NSCLC patients (3-6), leading to FDA approvals of three agents (nivolumab, pembrolizumab, and atezolizumab) for treatment of metastatic NSCLC patients. Other agents that also target this pathway, such as durvalumab (7) and avelumab (8), are currently under clinical development.
In an illustration of how these drugs work to eliminate tumor cells (Figure 1), the two most prominent actors are tumor cells themselves and tumor infiltrating cytotoxic T cells (1). In this context, tumor cells that express PD-L1 suppress immune reactions of PD-1 positive activated T cells through the PD-L1/PD-1 interaction, thus the blockade of this pathway by immunotherapeutic drug(s) enables T cells to counterattack tumor cells.
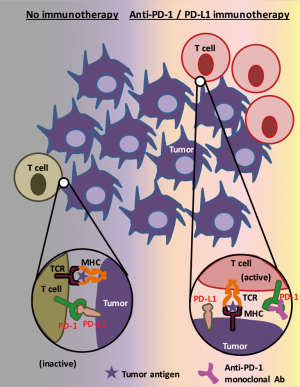
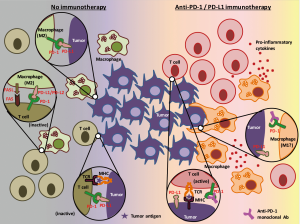
On the other hand, it is also true that tumor immune microenvironment not only contains cytotoxic T cells but also consists of heterogeneous cell populations (9) including natural killer cells, dendritic cells, regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages (TAMs). Several studies have identified that PD-1 is expressed in some of these immune cells other than cytotoxic T cells, and, reportedly, the PD-1 inhibits function of these immune cells (10-12). However, the roles of PD-1, expressed by these immune cells, on tumor maintenance and tumor development are not fully understood. In addition, it is also unclear to date how immunotherapies that target PD-1 or PD-L1 affect these PD-1 positive immune cells (other than T cells). In a recent study, Gordon et al. has reported that both mouse and human TAMs express PD-1, PD-1 expression negatively correlates with phagocytic potency of TAMs, and blockade of PD-1/PD-L1 pathway in vivo reduced tumor growth and lengthened the survival of mice in macrophage dependent fashion using in vitro and in vivo colon cancer models (13).
Macrophages are among the most abundant normal cells in the tumor microenvironment (14). Most tissue macrophages arise from yolk sac/fetal liver progenitors, and aside from that, the lung residential macrophages are from three distinct lineages (yolk sac/fetal liver/and bone marrow) that arrive at different times, reside in different locations (alveolar/interstitial, or peripheral/central), according to a recent lineage-tracing study in mice (15). On the other hand, macrophages involved in pathogen responses appear to come from circulating bone marrow monocytes (14).
When considering the roles of macrophages in tumors, although their functions are exceptional diverse, researchers often simply divide microphages into two subtypes; M1 macrophages (classically activated macrophages) and M2 macrophages (alternatively activated macrophages) (16). These subtypes correspond to “tumor killing” and “tumor promoting”, respectively, since M1 macrophages have roles to promote inflammation, while M2 macrophages suppress inflammation and facilitate tissue repair.
In clinical research settings, the roles of TAMs, as a prognostic factor, have been extensively studied in many tumor types, including NSCLCs. Although the results still have some contradictions, recent two meta-analyses (17,18) conclude that TAMs in tumor islet (higher density of M1 TAMs) predict better prognosis, while TAMs in stroma (higher density of M2 TAMs) predict worse prognosis in NSCLCs. These results suggest a dual role of TAMs in tumor development and maintenance, providing a rationale to target immune suppressive TAMs (M2 TAMs) as a part of immunotherapies for cancers. In fact, for patients with solid malignancy or lymphoma, there are several ongoing trials (e.g., NCT02216409) that target CD47, an “immune checkpoint” of macrophages, the inhibition of which facilitates macrophages to phagocyte tumor cells.
In the study by Gordon et al. (13), colon cancer mouse cell lines were subcutaneously injected into immunocompetent mice to assess the expression and the roles of PD-1 on TAMs. They observed that around 50% of macrophages in the tumors expressed surface PD-1 (which correlated with the time after engraftment and the tumor volume), whereas no circulating monocytes or splenic macrophages expressed detectable levels of PD-1. These PD-1 positive TAMs expressed an M2-like surface profile, and these findings were confirmed in human colon cancer specimens. The authors also found, by using a bone marrow transplantation mice model, that the time-dependent increase in PD-1 positive TAMs is mainly attributed to bone marrow-derived macrophages homing to the inflammatory tumor microenvironment, rather than from tissue-resident macrophages differentiating into PD-1 positive TAMs. Ex vivo phagocytosis assay with GFP+Staphylococcus aureus bioparticles and in vivo experiments with a mice model lacking an adaptive immune system revealed that PD-1 positive TAMs showed reduced degree of phagocytosis, compared to their PD-1 negative counterparts. The in vivo model also showed that the phagocytic ability of PD-1 positive TAMs further decreased if co-existent tumor cells expressed PD-L1. These results led the authors to perform experiments to treat mice (which also lack an adaptive immune system) with PD-L1 positive tumors by anti-PD-1, anti-PD-L1, or combination of anti-PD-L1 and anti-CD47 agents. As they expected, these immunotherapies showed efficacy over PBS control in terms of tumor volume and survival of mice. This novel finding may enhance our knowledge about the roles of the PD-1/PD-L1 pathway in the tumor immune microenvironment involving tumor cells, cytotoxic T cells, and TAMs (Figure 2).
The first question for this study is whether or not these results can be applied to NSCLC patients. Since these experiments utilized mouse models with subcutaneous injection of cancer cells, they may not have site-specific features (such as colon-specific or lung-specific) of the tumor immune microenvironment. In addition, a recent large scale comprehensive genomic study reported that the significantly mutated genes in lung adenocarcinomas were most similar to those in glioblastoma and colorectal cancer (19). Therefore, I propose that the roles of PD-1 positive TAMs, and the effects of anti-PD-1/anti-PD-L1 drugs on these TAMs in NSCLC patients are worthy of investigation in future studies, at least in lung adenocarcinoma patients.
Among lung adenocarcinoma patients, we now know that lung cancers with epidermal growth factor receptor (EGFR) activating mutations respond poorly to anti-PD-1/anti-PD-L1 drugs compared with lung cancers with wild-type EGFR (20,21). Lower immunogenicity due to lower mutation burden [higher mutation burden is one of potential predictive markers for higher efficacy of anti-PD1/anti-PD-L1 drugs in lung cancers (22)] in lung cancers with EGFR mutations may be one of explanations for these clinical observations. However, it is of note that a recent study that analyzed the correlation between intra-tumoral immune cell densities and genetic alterations in lung adenocarcinomas found that intra-tumoral macrophage density was significantly lower in tumors with EGFR mutations compared with those with wild-type EGFR (23). In this study, the density of neutrophils was also lower in lung cancers with EGFR mutations, while the densities of CD8 positive T-cells (cytotoxic T cells) and mature dendritic cells were identical. It is possible that lower density of intra-tumoral macrophages in lung cancers with EGFR mutations is one of additional mechanisms behind the lower efficacy of current immunotherapies in lung cancer patients with EGFR mutations.
The mechanisms of action of small molecule molecular targeted agents (e.g., EGFR tyrosine kinase inhibitors) are simple, since these drugs target molecular aberration(s) found only in tumor cells. In contrast, the target molecules of immunotherapies, i.e., immune checkpoint molecules, are shared by multiple types of immune cells/non-cancerous cells/tumor cells, and these cells interact with each other. Understanding the complexity of the tumor immune microenvironment may be essential to optimize and to personalize immunotherapies, as well as to precisely predict their efficacies and/or adverse events.
Acknowledgements
This work was supported by a grant from the International Association for the Study of Lung Cancer Young Investigator Award to K Suda (2015–2017).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [Crossref] [PubMed]
- Soo RA, Stone ECA, Cummings KM, et al. Scientific Advances in Thoracic Oncology 2016. J Thorac Oncol 2017;12:1183-209. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [Crossref] [PubMed]
- Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765-72. [Crossref] [PubMed]
- Benson DM Jr, Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010;116:2286-94. [Crossref] [PubMed]
- Karyampudi L, Lamichhane P, Krempski J, et al. PD-1 Blunts the Function of Ovarian Tumor-Infiltrating Dendritic Cells by Inactivating NF-¦ÊB. Cancer Res 2016;76:239-50. [Crossref] [PubMed]
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495-9. [Crossref] [PubMed]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49-61. [Crossref] [PubMed]
- Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development 2016;143:1318-27. [Crossref] [PubMed]
- Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 2012;32:463-88. [Crossref] [PubMed]
- Mei J, Xiao Z, Guo C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 2016;7:34217-28. [Crossref] [PubMed]
- Wu P, Wu D, Zhao L, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget 2016;7:40451-60. [Crossref] [PubMed]
- Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 2016;48:607-16. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Mansuet-Lupo A, Alifano M, Pécuchet N, et al. Intratumoral Immune Cell Densities Are Associated with Lung Adenocarcinoma Gene Alterations. Am J Respir Crit Care Med 2016;194:1403-12. [Crossref] [PubMed]


