Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version)
Bronchial asthma (hereinafter referred to as asthma) is one of common chronic respiratory diseases, and its global prevalence has been on the rise over the recent years. Clinical studies and practices show that standardized diagnosis and treatment, plus practically effective management, are important to achieve better asthma control and quality of life. The Asthma Workgroup, Chinese Thoracic Society, revised the Chinese Guidelines for Prevention and Treatment of Bronchial Asthma (hereinafter referred to as the Guidelines) twice, in 2003 and 2008. Overall, the Guidelines contributed substantially to the progress of prevention and treatment of bronchial asthma in China. However, owing to a variety of factors (such as shortage in measurement devices and asthma medications, under-developed system of medical education) in primary health-care institutions, the Guidelines have not been well adapted and fully implemented in these settings which are believed to be the vanguards in asthma prevention and treatment. In this regard, there is a dire need to release a primary health care version of these Guidelines which are well compatible with specific practices at community-level medical settings in China.
The Asthma Workgroup, Chinese Thoracic Society, in collaboration with the CMA Society of General Practitioners, has convened a panel of experts in related fields to discuss and develop a version of the Guideline for primary health care professionals (Primary Health Care Version) with the aim to standardize the diagnosis and treatment of asthma and to raise the awareness of asthma prevention in the community level.
1 Definition of asthma
Bronchial asthma is a chronic inflammatory disease of the airways, which is associated with the development and progression of airway hyperresponsiveness (AHR). Asthma results from a combination of genetic and environmental factors. Clinically, it is characterized by recurrent attacks of wheezing, shortness of breath, chest tightness and coughs, which frequently occur and aggravate at night or in the morning. In most patients, asthma can be controlled on medications.
2 Diagnosis
2.1 Diagnostic criteria
- Patient complaints of recurrent wheezing, shortness of breath, chest tightness and coughs, which are frequently associated with exposure to allergens, cold air, physical or chemical triggers, and with upper airway infections or physical exertion;
- Scattered or diffuse wheezing, typically during the expiratory phase, heard over the both lungs;
- These signs and symptoms may resolve spontaneously or with treatment;
- Other causes of wheezing, shortness of breath, chest tightness, and coughs are excluded;
- In patients with atypical clinical presentations (e.g., no symptoms or signs of wheezing), the diagnosis of asthma can be made based on any of the following: (i) peak expiratory flow (PEF) with a diurnal variation ≥20% as determined by a mini peak flow meter (see Appendix 1); or (ii) bronchial provocation test with a change of ≥12% and ≥200 mL in forced expiratory volume in 1 second (FEV1) (see Appendix 2).
Any subject who meets criteria items 1 through 4 or criteria items 4 plus 5 may be diagnosed with bronchial asthma.
2.2 Clinical stages of asthma
- Acute exacerbation of asthma;
- Chronic persistent asthma;
- Clinical remission of asthma;
Acute exacerbation of asthma is defined as a sudden attack of symptoms including wheezing, shortness of breath, chest distress and cough, or an aggravation of the pre-existing conditions, commonly associated with dyspnea and characterized by reduced expiratory flow. These events are often triggered by exposure to allergens or irritants, or respiratory infections.
Chronic persistent asthma is defined as the stage when subjects experience symptoms (e.g., wheezing, shortness of breath, chest tightness, and coughs) of varied frequency and/or severity each week.
Clinical remission of asthma is defined as resolution of the signs and symptoms either spontaneously or after treatment, with a pulmonary function that has been restored to the levels before exacerbation and maintained for at least 3 months.
2.3 Assessment of asthma
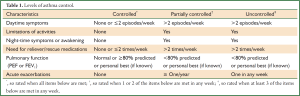
- Levels of asthma control (Table 1).
- Severity of asthma exacerbations: an episode of acute exacerbation varies in severity. In some cases, the disease can gradually worsen over several hours or a couple of days, whereas in severe cases, an acute exacerbation can become life-threatening within minutes. In order to provide a prompt and effective therapy, the asthma severity should therefore be properly assessed in each individual case (Table 2).
Full Table
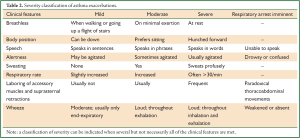
Full Table
2.4 Additional tests
- Pulmonary function tests: (i) Measurement of pulmonary ventilation: this test is considered as one of the most useful tests in confirming the diagnosis of asthma and assessing the level of asthma control, recommended for use in hospitals or institutions where such a test is available; (ii) Measurement of variation in PEF: this test is used to confirm the diagnosis of asthma and assess the level of asthma control in patients with atypical asthma (Appendix 1). Variation in diurnal PEF can be determined with a mini peak flow meter; (iii) Bronchial provocation test: this test is used to determine the presence of airway hyperresponsiveness. It is recommendable that patients with atypical presentations be referred to hospitals or institutions where bronchial provocation test is available, so as to confirm the diagnosis of asthma; (iv) Bronchodilation test: this test is used to determine the reversibility of airflow obstruction, which supports the diagnosis of asthma (Appendix 2).
- Skin prick test: sensitization to allergens among asthmatic patients can be confirmed by skin prick tests. The test can be used to identify the risk factors responsible for the development and exacerbation of asthma and to screen patients who might respond to specific immunotherapy.
2.5 Differential diagnosis
The differential diagnosis of asthma importantly includes primary left ventricular dysfunction, chronic obstructive pulmonary disease (COPD), and upper airway obstruction (Table 3). Other disorders include bronchiectasis, allergic granulomatosis (Churg-Strauss syndrome), and allergic bronchopulmonary aspergillosis (ABPA).
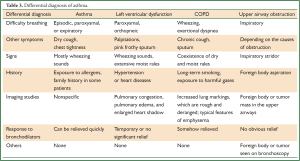
Full Table
3 Common medications for asthma: a brief introduction
Asthma medications are typically divided into two major categories--the controllers and the relievers. The controller medications are agents that inhibit airway inflammation and prevent asthma attack, prescribed for long-term use on a daily basis. Inhaled corticosteroids (ICS) are the drugs of first-line choice in this category, whereas others may include leukotriene modifiers, long-acting β2-agonists (only in combination with ICS), sustained-release theophyllines, and sodium cromoglycate. The reliever medications are prescribed for “as-needed” use, which may quickly relieve bronchospasm and wheezing. Inhaled rapid-acting β2-agonists are the first-line treatment in this category, while other drugs may include systemic glucocorticoids, inhaled short-acting anticholinergics, theophyllines, and oral β2-agonists. Commonly prescribed asthma medications and their mechanism of action, dosage, and precautions associated with their use are presented in Table 4.
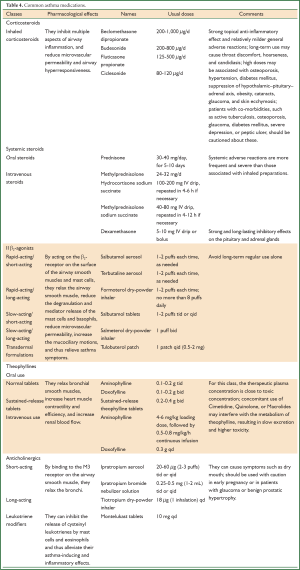
Full Table
Additional treatments for asthma include antihistaminic and antiallergic agents as well as those used in traditional Chinese medicine. Oral administration of antihistaminic and antiallergic agents, such as ketotifen, loratadine and tranilast, offers antiallergic and mild antiasthmatic effects which may favor the treatment of allergic asthma. Drowsiness is one of the most common adverse reactions of these agents. Etiology-based recipes of traditional Chinese medicine may be attempted as they are effective in alleviating asthmatic symptoms for patients during the clinical remission. However, physicians and patients should be alerted against the so-called “ancestral arcanum” or “empirical formula” which is alleged as “radical treatment of asthma” and remains popular among Chinese folks. A majority of such recipes unscrupulously contain unknown doses of oral corticosteroids. Despite temporary efficacy, their use might hamper effective treatment of asthma, cause corticosteroid dependence and serious adverse reactions, and should be therefore rejected.
4 Long-term maintenance therapy
4.1 Treatment goals
The goals of long-term treatment for asthma are to achieve and maintain symptom control; to maintain normal activity including physical exercise; to maintain a nearly normal pulmonary function; to prevent asthma exacerbations and adverse reactions related to asthma medications; and to reduce mortality associated with asthma.
4.2 Treatment strategies
- Selection of treatment regimens;
- Strategies for adjusting the treatment regimens;
- When to step up and down the treatment regimen;
Selection of treatment regimens for asthma should be based on the assessment of disease severity and the level of control. Decision-making on asthma medications should take into consideration the efficacy and safety of drugs, and also their affordability and accessibility for the patient and health care institution. Personalized treatment and follow-up protocols should be established for each new case of asthma, in order to ensure follow-up and monitoring on a regular basis and to achieve good patient compliance. Timely adjustment to the treatment regimen would be needed along with the patient’s condition. The long-term treatment of asthma is divided into five steps (Table 5).
For most patients newly diagnosed with mild asthma or not yet on medication, treatment should be started at Step 2. For those who are obviously symptomatic, start the treatment at Step 3 with low-dose ICS plus sustained-release theophylline as the recommended option, pending the use of low-dose ICS plus long-acting β2-agonists (preferably aerosols) or leukotriene modifiers to be considered in primary health care institutions where these medications are available. Similarly, moderate-to-high doses of ICS plus theophylline should be the first choice for Step 4 treatment. ICSs are the main controller in Steps 2 to 5. In any step of treatment, reliever medications should be used as needed for a quick relief of asthma symptoms.
If asthma is not controlled on the current treatment regimen, step up until the asthma control is achieved. The efficacy and safety of the regimen containing lowest-dose oral corticosteroid plus oral sustained-release theophylline should be investigated in further clinical trials. In particular, long-term use of oral corticosteroids may cause general adverse effects that should warrant careful monitoring.
Stepping down of the treatment may be considered if asthma control has been achieved and maintained for at least 3 months. Recommendations for stepping down are as follows: (i) when moderate-to-high-dose ICS alone is being used, a 50% reduction in the dose could be attempted; (ii) when control is achieved at a low dose of ICS alone, the regimen could be switched to once-daily dosing; (iii) when asthma is controlled with a combination of ICS and oral sustained-release theophylline, consider reducing the dose of ICS by approximately 50% while continuing the sustained-release theophylline. If asthma control has been maintained for 1 year with lowest dose of controller medications and no asthma symptoms have been reported, discontinuation of asthma medications may be considered.
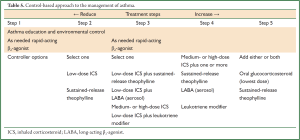
Full Table
5 Treatment of asthma exacerbations
5.1 Treatment goals
The treatment goals for asthma exacerbations are to relieve airflow obstruction, resolve symptoms, and improve hypoxia as soon as possible.
5.2 Treatment strategies
Removal of predisposing factors, relief of bronchospasm and asthma symptoms, correction of hypoxia, sufficient and proper use of systemic corticosteroids.
5.3 Managing asthma exacerbations
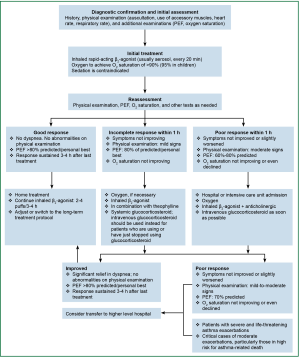
- Confirming the diagnosis and assessing the severity: on presentation of a suspected patient, a medical history should be taken, physical examination performed, and PEF and pulse oxygen saturation measured, to establish a definitive diagnosis and assess the severity of the exacerbation. Remove or avoid any predisposing factors that could be identified as soon as possible, including a polluted environment, allergen exposure, or use of nonsteroidal anti-inflammatory drugs. Any active infections should be effectively controlled.
- Medications: the following aspects should be emphasized: (i) For patients recently not on theophylline, a load dose (4-6 mg/kg) of theophylline could be administered first by slow intravenous injection over a duration of more than 20 min, followed by intravenous dripping of a maintenance dose (0.6-0.8 mg/kg–1/h–1); for patients who have experienced adverse reactions related to theophylline, doxofylline (0.2 g/12 h IV or 0.3 g/d IV gtt) could be use alternatively because it has a better safety profile. (ii) Recommended glucocorticoids for systemic use include hydrocortisone sodium succinate, prednisone, prednisolone, and methylprednisolone. Dexamethasone is usually not recommended because of its long-lasting actions and potent inhibitory effects on the hypothalamic–pituitary–adrenal axis, but can be considered when and where other systemic glucocorticoids are not available. Patients with mild asthma can be given oral prednisone or prednisolone (0.5-1 mg/kg–1/d–1). For those are currently on or have recently discontinued oral glucocorticoids, a switch from oral to intravenous administration should be considered. In these patients, hydrocortisone sodium succinate (10 mg/kg–1/d–1, calculated as hydrocortisone equivalent), methylprednisolone (40-80 mg/d given in divided doses), or dexamethasone (0.1-0.2 mg/kg–1/d–1) can be administered. In some patients, oral medications could be sequentially administered for a treatment course of 5 to 7 days after asthma control has been achieved. (iii) Combination of inhaled β2-agonists and anticholinergics may produce more favorable bronchodilatory effects. Generally, the recommended combination is inhalation of salbutamol 2.5 mg or terbutaline 5 mg plus ipratropium bromide 0.25 mg, delivered every 6 to 8 h.
- Criteria for referral to a higher level hospital: (i) Mild to moderate exacerbation with poor treatment response or even worsening of the condition despite above-mentioned medications for at least 24 h; (ii) critical cases of moderate exacerbations, in particular, patients with high risk of asthma-related death; and (iii) severe or extremely severe episode of asthma exacerbation as classified at initial severity assessment. Patients who meet criteria (ii) or (iii) should be given emergency treatment before they can be transferred to a higher level hospital immediately after their condition becomes stable. En route, accessibility to first-aid measures such as oxygen supply, intravenous access, and endotracheal intubation should be guaranteed.
- Treatment algorithm for asthma exacerbations: see Figure 1.
6 Prevention and health management of asthma
6.1 Goals
The goals for prevention and health management of asthma are to help patients identify and avoid risk factors related to development and progression of asthma, improve their awareness and capacity of self-care, modify their lifestyles, prevent asthma exacerbation, enhance their compliance and adherence to medical advices, achieve and maintain asthma control, and reduce future risks associated with their condition. Community-based asthma education programs are complementary to hospital-based medical services. Prevention and health-caring of asthma at the community level should be integrated with the treatment and diagnosis provided by general practitioners.
6.2 Establishing a partnership between patients and physicians
A personalized treatment protocol, which covers self-monitoring, should be tailored for each patient. A good patient-physician partnership would facilitate communication and medical education, and would engage the patients in management and control of asthma under physician’s instructions.
6.3 Reducing risk factors
Patients should be instructed to avoid or reduce exposure to indoor or outdoor allergens, offending viruses, pollutants, tobacco smoke or certain drugs, so as to prevent onset or worsening of asthma.
6.4 Long-term management
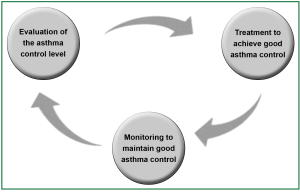
Over the long-term follow-up, level of asthma control in patients should be assessed according to the criteria stated above. In all patients, asthma control should be achieved and maintained by escalating or de-escalating to proper treatment steps. Down-stepping of treatment regimens may be considered only after maintenance of asthma control for at least 3 months. Consider stepping-up for failed control of asthma or onset of acute exacerbation until asthma control is achieved.
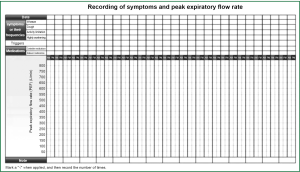
A follow-up visit is generally scheduled every 1 to 3 months for patients even though with achieved asthma control; but for those who have a recent exacerbation, every 2 to 4 weeks. During the visits, data on home PEF measurement and symptom records, acquired skills for inhalation, risk factors, and the level of asthma control should be collected. Patients should be encouraged to keep an asthma diary which includes daily symptoms, twice-daily measurements of PEF, and outcomes of asthma control test (ACT) which is performed once every 4 weeks. In addition, the patients should be followed up for maintenance of asthma control, prompt adjustment of treatment regimens and the need to reduce dosing of controllers (Figure 2).
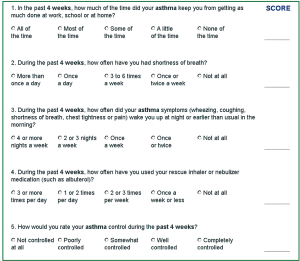
- ACT. ACT is a test which provides comprehensive evaluation of asthma control simply based on the total score of individual patient response to a 5-item questionnaire about asthma symptoms and quality of life (Figure 3). A score of 25 points indicates controlled asthma; 20 to 24, partially controlled; and 19 or below, uncontrolled. The ACT is complementary to the pulmonary function test, and can be used for the self-assessment of asthma control level either completed at home or in a community survey. This measurement may help to achieve a better assessment of asthma control and improve the interaction between physicians and patients. It also provides reproducible objective parameters for long-term monitoring. Continuous monitoring of these parameters may be critical in adjusting the treatment regimen and determining the minimum treatment step that is sufficient to keep asthma under control, and hence at a lower medical cost.
- Interpretation of PEF measurement. Normal (green zone): you are safe when PEF >80% of predicted value or your personal best, with a diurnal variation below 20%; Alert (yellow zone): you are at risk of asthma attack when PEF is between 60% and 80% of predicted value or your personal best, with a diurnal variation between 20% and 30%. Danger (red zone): you need prompt medial aid or an immediate visit to the emergency department when PEF is below 60% of predicted value or your personal best.
- Asthma education. The basic component of asthma health management is to provide education on asthma for community dwellers and asthma patients. On a regular basis, continued medical education delivered to general practitioners and other health care providers at primary care settings needs to address the topics on asthma care management and skills for physician-patient interaction, so as to ensure the efficiency of physician-initiated education for patients and their families. Ultimately, asthma education aims to render better understanding and knowledge of the disease, to improve health-caring, to increase patient satisfaction and their confidence, compliance, and self-management, as well as to reduce medical costs.
Asthma education should cover the following areas: (i) the goals of asthma treatment, namely, achieving effective and sustained asthma control by long-term standardized treatments; (ii) the etiology and underlying mechanism of asthma; (iii) avoidance of risk factors; (iv) long-term asthma treatment; (v) introduction of inhalers and instructions for manipulation; (vi) self-monitoring: how to keep and interpret an asthma diary (see Figure 4 for a sample sheet of asthma diary), including symptom scores, medication use, PEF measurements, and results of the ACT; (vii) signs of an impending attack, presentations of asthma, what to do and when to see a doctor; (viii) knowledge about asthma medications; (ix) how to assess asthma control levels and choose proper regimen based on self-monitoring; and (x) the role of mental factors in the development of asthma.
Asthma education is delivered in several modalities: (i) patient education at initial diagnosis—this is the most important because it marks the beginning of a partnership between the patient and physician. At this stage, asthma education should be individualized to a patient’s needs and cover the most elementary issues. First, the patient should be informed of the diagnosis, and enquired about and evaluated for their expectations of medical treatment. At the least, the first 6 topics of asthma education described above should be communicated to the patient. Follow-up visits are scheduled and free educational materials delivered to the patient as well. (ii) Follow-up education and evaluation: all questions from the patients should be answered and the level of asthma control assessed during each follow-up visit. (iii) Group education, referring to asthma education events taking place on a regular basis at the community level, where face-to-face patient-physician interviews as well as question-and-answer sessions are available. (iv) Self-education through free distribution of brochures on the prevention and treatment of asthma. (v) Mutual learning, as accessible in community-based asthma clubs and experience-sharing sessions.
All in all, asthma education is a long-term ongoing project which requires regular updates and perseverance.
Appendix 1. Simple PEF measurement and calculation of diurnal variation
Procedure
Instruct patient to stand upright. Take several normal breaths, followed by a deep inspiration to achieve the total lung capacity. Place a simple peak flow meter into the mouth and exhale as hard and fast as possible to achieve the residual capacity. Record the reading of PEF value as indicated by the spirometer. The procedure should be repeated at least 3 times at an interval of 5 to 10 min. Record the highest PEF of the three readings on the spirometer as the result of a PEF measurement. On a daily basis, PEF is measured twice, one in the morning and one at the bedtime. Diurnal variation in PEF is calculated after consecutive measuring for at least one week.
Calculation
Diurnal variation of PEF = (Max - Min)/[(Max + Min)/2] ×100%, where Max (or Min) denotes the greater (or smaller) PEF value measured on a given day.
Criteria for positive results: diurnal variation ≥20%
Clinical relevance
- A confirmed diagnosis in patients with atypical asthma can be determined when diurnal variation of PEF ≥20%.
- Evaluation of asthma severity: In many cases, asthma occurs or worsens at night or in the morning. Therefore, routine daily PEF measurements may help to reveal diurnal variations in PEF and allow for assessment of asthma severity. An increasing trend in PEF variation and/or a progressively decreasing trend in the PEF curve may indicate a risk of acute onset or asthma exacerbation.
Appendix 2. Bronchodilation test
Procedure
First, the patient undergoes measurement of baseline (pre-bronchodilator) FEV1 or PEF. Then the patient receives inhalation of 200 to 400 μg salbutamol or other rapid-acting β2-agonists via a metered-dose inhaler (MDI). The procedure is repeated at 15 to 20 min after inhalation to obtain the value of post-bronchodilator FEV1 (or PEF).
Calculation
Improvement in FEV1 (or PEF) = [post-bronchodilator FEV1 (or PEF) – pre-bronchodilator FEV1 (or PEF)]/[pre-bronchodilator FEV1 (or PEF)] ×100%
Criteria for positive results: improvement in FEV1 ≥12% and ≥200 mL.
Clinical relevance
Bronchodilation test can be used to assess the reversibility of airflow limitation, confirm the diagnosis of asthma, and to evaluate the response to bronchodilator treatment.
Acknowledgements
These guidelines were translated from a Chinese version by Dr Prof Guangqiao Zeng and his colleagues, with permission and authorization from Prof Jiang-Tao Lin. The translators aim to promote and distribute these guidelines to a wider international scientific audience, and declare no conflict of interest. Contributors of these guidelines are (sort by chapter): Jiang-Tao Lin (China-Japan Friendship Hospital), Shan-Zhu Zhu (Zhongshan Hospital, Fudan University), Jia-Ji Wang (School of Public Health, Guangzhou Medical College), Nan Su (China-Japan Friendship Hospital), Yi-Qiang Chen (First Affiliated Hospital, Guangxi Medical University), Kai-Sheng Yin (First Affiliated Hospital, Nanjing Medical University), Xin Zhou (First People’s Hospital, Shanghai Jiaotong University), Chang-Gui Wu (Xijing Hospital, Fourth Military Medical University), and Ping Chen (General Hospital of Shenyang Military Region). The guidelines are endorsed by a panel of experts (in alphabetical order): Shao-Xi Cai (Nanfang Hospital, Southern Medical University), Ping Chen (General Hospital of Shenyang Military Region), Yi-Qiang Chen (First Affiliated Hospital, Guangxi Medical University), Ling-Fei Kong (First Affiliated Hospital, China Medical University), Mao Huang (First Affiliated Hospital, Nanjing Medical University), Jing Li (Guangzhou Institute of Respiratory Disease), Jiang-Tao Lin (China-Japan Friendship Hospital), Ao Liu (Kunming General Hospital of Chengdu Military Region), Chun-Tao Liu (West China Hospital, Sichuan University), Rong-Yu Liu (First Affiliated Hospital, Anhui Medical University), Xian-Sheng Liu (Tongji Hospital, Central China Medical University), Chen Qiu (Shenzhen People’s Hospital), Hua-Hao Shen (Second Affiliated Hospital Zhejiang University School of Medicine), Nan Su (China-Japan Friendship Hospital), Yong-Chang Sun (Beijing Tongren Hospital, Capital Medical University), Huan-Ying Wan (Ruijin Hospital, Shanghai Jiaotong University), Chang-Zheng Wang (Xinqiao Hospital, Third Military Medical University), Chang-Gui Wu (Xijing Hospital, Fourth Military Medical University), Wen-Bing Xu (Peking Union Medical College Hospital), Ya-Dong Yuan (Second Affiliated Hospital, Hebei Medical University), Kai-Sheng Yin (First Affiliated Hospital, Nanjing Medical University), and Wei-He Zhao (Ningbo No. 2 Hospital).