A young woman with a giant breast fibrosarcoma: a case report
Introduction
Breast fibrosarcoma is a rare tumor. It is one of breast sarcomas which are rare, and account 0.1% of malignant breast tumours (1). In this case, we discuss a patient who developed a rapidly expanding malignant breast fibrosarcoma with a diameter of about 30 cm. The early age of onset and rapid growth make this case special.
Case presentation
It was a 15-year-old girl who came to our department with her parents. She told us it had been 5 months since the lump occurred in her right breast. The lump grew gradually and she didn’t felt any pain or discomfort. So they didn’t take it seriously until one month ago. The lump grew rapidly and the color of her breast became dark red.
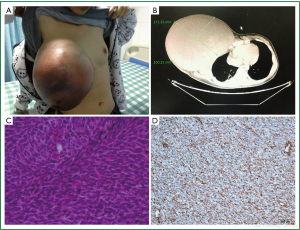
When we did a physical examination for her, we were shocked by the size and color of her right breast. It was 30 cm × 30 cm × 10 cm large that can reach the level of the navel when she stood up and half of it was dark red. Her quite thin skin of right breast and engorgement of right breast superficial vein can be obviously seen (Figure 1A). But no axillary lymph node was palpable. The examination of the left breast was normal. We gave her blood test, ultrasound test and computed tomography (CT) scan. The results of ultrasound and blood test were not specific for diagnosis. It was indicated by the CT that there were pathological fractures in the third and fourth ribs and the radiology doctors presumed that it was due to the tumor’s invasion (Figure 1B). Fine-needle aspiration result suggested that it might be a phyllodes tumor of the breast. So we performed an expended resection on her, with the scope including the whole right breast, the right pectoralis major muscle and the right pectoralis minor muscle without axillary lymph nodes dissection. HE Staining (Figure 1C) and immunohistochemistry results are as follow: Vim (+) (Figure 1D), CD99 (+), Bc12 (+), EMA (+/–), Ki67 (50%+), SMA (–), S100 (–), HHF35 (–), Des (–), Myogenin (–), Myoglobin (–), CD117 (–), CD34 (–). It was suggested that the lump was a mesenchymal tumour and was highly malignant. But it’s not yet to make an exact diagnosis. So we did a fluorescent in situ hybridization (FISH) of Synaptotagmin gene of the tumour. The result was negative with which synovial sarcoma could be excluded. The finally diagnosis was breast fibrosarcoma. However, they refused to continue chemotherapy for fear of its side effects. We found no recurrence of the tumor after a month of follow-up and then we lost to follow-up.
Discussion
Fibrosarcoma, as a subtype of soft tissue sarcoma, originates from fibroblasts, and has been defined as the production of collagen fibers sarcoma which is a common diagnostic term in the 50s and 60s of the 20th century. With increasingly stringent criteria and diagnostic methods, most of which had been diagnosed as fibrosarcoma are classified as synovial sarcoma or other diseases now.
Breast fibrosarcoma is one of the sarcomas of the breast. Pollard et al. reported that there were 4 cases of fibrosarcoma among the 25 cases of breast sarcoma (2). And according to Surov et al. and their clinical data, breast sarcoma was rare that account 0.1% of malignant breast tumors and the incidence of breast fibrosarcomas is about a quarter of that (1,3). However, because of the low incidence, there is no exactly figure of that.
Patients typically present with a single, round or oval-shaped, painless lump. Generally, it’s smooth, mobile, well circumscribed and not involving the nipple. It is more than 5 cm in diameter, located in the center of breast except giant lumps which can occupy the entire breast. The lump exhibits continuous growth, but it may also increase rapidly in size. As a result of stretching and increased pressure, the attenuated skin ulcerates secondary to ischemia.
Ultrasonography shows that, it is a nodular, well-circumscribed hypoechoic, heterogeneous lesion, and the blood flow can be found. Cystic echo can be seen in tumor. Most of Cystic echo is due to hemorrhage, necrosis and mucoid degeneration. CT scan has been widely used in the diagnosis of soft tissue sarcoma, providing tumor size, location and adjacent tissues and organs and anatomical relationship in recent years. MRI has also been more and more used. Comparing with CT, MRI has more significance in detecting the relationship between tumors and surrounding tissue.
Pathologically, gross appearance of the tumor often shows that it has clear boundaries and is round or oval. The differentiated sections are gray with hard and tough texture. While the poorly differentiated sections are fish-meat like with soft texture, and even with hemorrhage, necrosis or cystic degeneration. Endoscopically, both of them are constituted by relatively homogeneous spindle cells. These cells have ill-defined and lightly stained cytoplasm, fusiform nuclei, sharp ends and are arranged in a typical Sapphire osteoid bundle structure. The number of mitotic figures varies while the cell morphology is consistent.
However, Fletcher believed that breast fibrosarcoma is a diagnosis by excluding of other diseases. To this patient, we found it difficult to be distinguished from synovial sarcoma and phyllodes tumor of the breast, according to medical history, symptoms, signs and accessory examinations. What’s more, histologically, synovial sarcoma is in naive mesenchymal cell formation with synovial differentiation. Typically, the synovial sarcoma cells diphasically differentiate into carcinoma-like epithelial cells and sarcoma-like spindle cells. Usually there is migration between them (4,5). Shih-ping Cheng believed that the smaller the tumor is, the more it’s like a breast fibroma pathologically; the larger, the more it’s like a sarcoma (6).
According to its definition, the tumour’s Vimentin I collagen is positive and S-100 protein, EMA (epithelial membrane antigen), keratin and binding protein staining are negative in immunohistochemistry. We excluded synovial sarcoma by Synaptotagmin gene (–). Endoscopically, phyllodes tumors are shown to have large fissures that make the tumor lobulated, with epithelial cells lining their inner walls, which is the most important point to tell them from fibrosarcomas.
The new AJCC staging system is based on tumor grades, size, and the relative position of the fascia and muscle. Soft tissue sarcoma is divided into four stages. Tumor grade G: 1 level: well-differentiated, 2 level: moderately differentiated, 3 level: poorly differentiated, 4: undifferentiated. Tumor size: T1 ≤5 cm, T2 >5 cm. The location of the tumor and chest myofascial: Ta: superficial fascia in the chest Tb: in chest deep fascia. Specific stage: stage I: I A: G1, 2, T1a, b, N0, M0; I B: G1, 2, T2a, N0, M0; stage II: II A: G1, 2, T2b, N0, M0; II B G3, 4, T1, N0, M0; II C G3, 4, T2a, N0, M0; stage III: G3, 4, T2b, N0, M0 ; stage IV: IV A: any G, any T, N1, M0; IV B: any G, any T, N, M1. According staging criteria, the patient belongs to stage III.
To breast fibrosarcoma, surgical resection is a preferred treatment currently. Extended resection of tumor and mastectomy are the major surgical procedures. The extent of the surgery is based on the preoperative histological and radiological tests results, including the skin, subcutaneous tissue, normal tissue 2-3 cm from the tumor margin and the wound of biopsy or drainage. To reduce the risk of local recurrence, the mastectomy may be considered for those whose tumors are too large. Prone to hematogenous metastasis rather than lymph node metastasis, soft tissue sarcomas differ with carcinomas. Moreover, Fong et al. reported that lymph node metastasis accounted for 2.7% in 1,772 cases of adult soft tissue sarcoma, including angiosarcomas (13.5%), embryonal rhabdomyosarcomas (13.6%), and epithelial sarcomas (16.7%). As fibrosarcomas have a significantly small proportion of that, conventional axillary lymph node dissection for breast fibrosarcoma is not necessary (7).
Radiotherapy is not a conventional treatment of fibrosarcoma due to its insensitivity to it. But it can be considered for inoperable patients, which may improve the outcome of those with hyperthermia. Kurkchubasche et al. believed that adjuvant chemotherapy in rhabdomyosarcoma, Ewing’s sarcoma and osteosarcoma and other diseases plays an important role, but not in fibrosarcoma (8). Adjuvant chemotherapy is worth a shot to patients with highly malignant sarcoma, with positive surgical margin or postoperative recurrence. The most effective chemotherapy regimen is adriamycin (ADM) + ifosfamide (IFO). The slope of dose-effect curves is a key factor to determine its effect. When the dose of doxorubicin is 45 mg/m2, the effective rate is less than 20%. However, when the dose is 75 mg/m2, the effective rate is 37%. And there is a similar dose-effect relationship in chemotherapy or in combination with other chemotherapy drugs. High-dose chemotherapy of AI is currently recognized as the most effective chemotherapy. A study of Italian cooperative group showed that, after a median follow-up time of 59 months of patients who were at stage III, large doses of epirubicin and IFO chemotherapy can improve overall survival and disease-free survival (9).
It is generally agreed that the prognosis of the disease is related with tumor cell differentiation, tumor size, and surgical approaches. Poorly differentiated tumors grow faster, the more prone to ulcers or hematogenous metastasis at early stages, the poorer prognosis. However, Adem et al. reported 25 cases of patients with breast sarcoma, in which after 10.5 years of follow-up, local recurrence was observed in 11 patients and DFS ranged from 2 to 36 months (mean 15 months), while distant metastasis was observed in 10 patients (40%) in lungs, bones, livers, spleens, and skin. Five-year survival was 66% and cause-specific survival (CSS) was 70%. DFS at 5 years were 91% for tumours size ≤5 cm and 50% for tumours size >5 cm. Tumour size was significantly associated with OS. There was no significant difference in OS or CSS between low- and high-grade lesions (3). Therefore they believe that, as prognostic factors, tumor size matters more than the tumor grade.
Conclusions
Breast fibrosarcoma is a rare tumor. The early age of onset and rapid growth make this case special. The patient was late for treatment, which is very unfortunate. We make the diagnosis through pathology, immunohistochemistry, and exclusion of other diseases. Surgical resection is a preferred treatment currently, with extended resection of tumor and simple mastectomy. Radiotherapy and chemotherapy can be options for patients with high-grade sarcomas, positive surgical margins or postoperative recurrence. The most effective chemotherapy is high-dose AI. As the disease is characterized by a poor prognosis and a susceptibility to recurrence, we are expecting more advanced technology and treatment to be involved in the disease.
Besides, the auxiliary examination was noticed to have some benefit to diagnosis, nevertheless by which a definite diagnosis cannot be made due to a certain rate of misdiagnosis.
Acknowledgements
We thank cancer center of Sun Yat-sen University for their support and consideration. We also thank Doctor Haoyu Peng of Nanfang Hospital, Southern Medical University for the revision of our report.
Disclosure: The authors declare no conflict of interest.
References
- Surov A, Holzhausen HJ, Ruschke K, et al. Primary breast sarcoma: prevalence, clinical signs, and radiological features. Acta Radiol 2011;52:597-601. [PubMed]
- Pollard SG, Marks PV, Temple LN, et al. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer 1990;66:941-4. [PubMed]
- Adem C, Reynolds C, Ingle JN, et al. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 2004;91:237-41. [PubMed]
- Cagle LA, Mirra JM, Storm FK, et al. Histologic features relating to prognosis in synovial sarcoma. Cancer 1987;59:1810-4. [PubMed]
- Doyle VJ, Bateman AC, Theaker JM. An unusual breast mass: primary synovial sarcoma. BMJ Case Rep 2013;2013. pii: bcr2013010468.
- Cheng SP, Chang YC, Liu TP, et al. Phyllodes tumor of the breast: the challenge persists. World J Surg 2006;30:1414-21. [PubMed]
- Fong Y, Coit DG, Woodruff JM, et al. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993;217:72-7. [PubMed]
- Kurkchubasche AG, Halvorson EG, Forman EN, et al. The role of preoperative chemotherapy in the treatment of infantile fibrosarcoma. J Pediatr Surg 2000;35:880-3. [PubMed]
- Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 2001;19:1238-47. [PubMed]


