The influence of less invasive ventricular assist device implantation on renal function
Introduction
Over the past several years, the long-term-survival of patients suffering from coronary heart disease has been considerably enhanced by improved pharmacological and surgical interventions (1). However, this has led to an increased prevalence of chronic heart failure (2), a symptomatic disease, accompanied by various other end-organ dysfunctions as a result of low cardiac output.
As the percentage of cardiac output delivered to the kidneys is 25% (3), the function of the kidneys is particularly dependent on blood circulation conditions. Chronic heart failure in conjunction with low cardiac output could therefore lead to so called ‘cardiorenal syndrome’ type 2, which is defined by cardiac abnormalities resulting in impaired kidney function as a result of hypoperfusion, venous congestion and inflammation (4-6). It is also known, that there is a direct correlation between the prognosis of heart failure patients and the grade of kidney dysfunction (7,8). The simultaneous development of both cardiac- and renal failure is associated with increased chances of mortality (9).
In case of end-stage chronic heart failure, cardiac transplantation is still the therapy of choice, although new treatment strategies have to be developed to overcome the ongoing insufficient donor organ supply. One promising alternative to address the aforementioned issue is the implantation of left ventricular assist devices (LVAD) which is one of the fastest-developing techniques in cardiac surgery, due consistent technological advancements allowing less invasive surgical techniques and miniaturization (10-13).
In contrast to the standard surgical approach (SAS) the less invasive implantation technique (LIS) is associated with reduced trauma, blood loss and infection rate (14) and should therefore lead to a better outcome in terms of kidney function.
Although the effects of conventional LVAD implantation on short- and long-term renal function (5,15) has been well studied, little information is available on the corresponding effects of less invasive surgical approaches.
In this study, we have therefore assessed the impact of LVAD implantation with a minimally invasive technique on the renal function of heart failure patients who underwent dialysis due to perioperative kidney failure.
Methods
We retrospectively analyzed the data of 103 patients (80 males, 23 females) with severe heart failure, who underwent ventricular assist device implantation at the Hannover Medical School and were dialyzed due to renal failure within a 15-year time period (2001–2016). Renal dysfunction was considered “at risk” [glomerular filtration rate (GFR)] >90 mL/min), “mild” (GFR =60–89 mL/min), “moderate” (GFR =30–59 mL/min), “severe” (GFR =15–29 mL/min) or “end stage” (GFR <15 mL/min).
Patient plasma creatinine- and urea-levels were assessed on the hospital admission and discharge dates. GFR was calculated using the MDRD (Modification of Diet in Renal Disease): 186 × Serum Creatinine−1.154 × Age−0.203 × [0.742; for female patients].
Statistical analysis of clinical data was performed using SPSS 20.0 (IBM SPSS Statistics, IBM Corp., Armonyk NY, USA). Comparison was made using two-sided paired t-test. Differences were considered significant at P<0.05. Data has been represented as mean ± standard deviation (SD).
This study was performed as per the principles outlined in the Declaration of Helsinki and approved by the local institutional review board.
Results
Out of 103 patients, 90 patients underwent conventional LVAD implantation (standard approach surgery, SAS) and 13 patients underwent less invasive LVAD implantation (less invasive surgery, LIS). The mean duration of SAS implantation was 220±77 minutes while that of LIS implantation was 207±39 minutes, mean hospital stay was 62±56 days. Kidney dysfunction was considered “at risk” in 11.7% (n=12), “mild” in 16.5% (n=17), “moderate” in 23.3% (n=24), “severe” in 32% (n=33) and “end stage” in 16.5% (n=17) of patients.
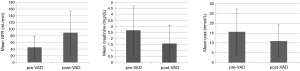
Overall mean preoperative renal parameters showed ‘moderate’ renal dysfunction with a calculated GFR of 45.01±33.95 mL/min. Preoperative mean creatinine level was 2.689±2.01 mg/dL and mean urea level was 15.62±11.62 mmol/L. Postoperative renal parameters showed normal renal function with a calculated GFR of 89.23±64.81 mL/min, a mean creatinine level of 1.58±1.51 mg/dL and a mean urea level of 11.00±8.13 mmol/L. Data analysis showed significant increase in GFR (44.2±56.48 mL/min; 95% CI: 33.81–55.28; P<0.001) and a significant decrease in creatinine (−1.08±1.83 mg/dL; 95% CI: 0.75–1.46; P<0.001) and urea (−4.62±13.66 mmol/L; 95% CI: 1.95–7.29; P<0.001) in all patients (Figure 1).
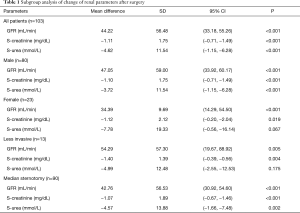
With the exception of the altered urea levels in female patients, there was also a significant improvement of renal parameters between male and female patients. Nevertheless, there was no significant difference in the increase of renal function between male and female patients (GFR: P=0.346; creatinine: P=0.965; urea: P=0.210).
Subgroup analysis revealed a significant improvement of renal function in both SAS (GFR: 42.76±56.53 mL/min; 95% CI: 30.92–54.60; P<0,001; creatinine: −1.07±1.89 mg/dL; 95% CI: −0.67 to −1.46; P<0.001; urea: −4.57±13.88; 95% CI: −1.66 to −7.48; P=0.002) and, except for urea, LIS (GFR: 54.29±57.30 mL/min; 95% CI: 19.67–88.92; P=0.005; creatinine: −1.40±1.39 mg/dL; 95% CI: −0.39 to −0.56; P=0.004; urea: −4.99±12.48; 95% CI: −2.55 to −12.53; P=0.175) (Table 1). However, there was also no significant difference in the increase of renal function between LIS and SAS (GFR: P=0.494; creatinine: P=0.543; urea P=0.918).
Full table
Discussion
Cardiac output is crucial for renal function: chronic heart failure accompanied by low cardiac output leads to decreased effective circulating volume as well as backward failure with a high venous pressure, which is directly transmitted to the renal tubular system. This leads to decreasing net filtration and therefore to a decreased GFR, resulting in an activation of the neurohumeral system (4,5,16). Hence a therapy for improvement of cardiac output could enhance renal function in heart failure patients.
In endstage chronic heart failure, the gold standard for therapy is still cardiac transplantation however the implantation of an LVAD is a promising alternative. Due to consistently improved technology, e.g., pump size miniaturization and less-LISs, the implantation of LVADs is one of the fastest-developing fields in cardiac surgery (10,17), thus the number of LVAD implantations worldwide has surpassed the number of cardiac transplantations (18). It is known that the major advantages of a less invasive surgical approach are reduction of trauma, reduced blood loss and less infections as well as decreased duration of intensive care unit and in-hospital stay (14,19), but little is known on the impact of LIS on renal function. The aim of our study was therefore the assessment of the relationship between LVAD implantation and its impact on kidney function with regard to minimally invasive surgery.
In our study, we found out that the overall implantation of an LVAD has a significant positive impact on renal function. The mean change of the GFR at the day of discharge was 44.2±56.48 mL/min (P<0.001), the mean change of creatinine level was −1.08±1.83 mg/dL (P<0.001) and of urea level was −4.62±13.66 mmol/L (P<0.001), in our patient cohort.
These results corroborate data from preceding investigations, as the impact of ventricular assist device on renal function has been well investigated (20-22). In summary, short term results show that the implantation of a continuous flow LVAD maintains or improves renal function.
A study conducted by Brisco et al. showed that there is a significant change of renal function in patients who underwent mechanical circulatory support. In their study, the median improvement of GFR in the first weeks was 48.9% (P<0.001), with 22.3% of the population improving ≥100% (23). However, they also stated that the improvements appeared to be transient: after one year of follow up, the GFR was only 6.7% higher than the pre-implant value (23). Further studies are therefore necessary to confirm the long-term effects of SAS ventricular assist device implantation and to assess the long-term results of LIS implantation in view of renal function.
The current study also revealed that with the exception of the mean change of urea in female patients, there was also a significant improvement of renal parameters, in a manner related to patient sex. Yet, there was no significant difference in the increase of renal function between male and female patients (mean difference of GFR: −12.65±13.37 mL/min (P=0.346); mean difference of creatinine: 0.02±0.44 mg/dL (P=0.965) and mean difference of urea: 4.06±3.22 mmol/L (P=0.210), although we expected a significant higher improvement of renal function in males patients, as female patients suffering from heart failure face a higher incidence of comorbidity with impact on renal function and a higher mortality (24,25).
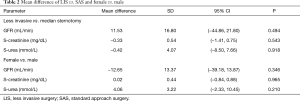
The present study demonstrated that as compared to a standard implantation technique, a less invasive approach may be associated with better kidney function outcome. The mean difference of GFR was 11.53±16.80 mL/min (P=0.494), the mean difference of creatinine was −0.33±0.54 mg/dL (P=0.543) and the mean difference of urea was −0.42±4.07 mmol/L (P=0.918) (Table 2). Due to aforementioned advantages of a less invasive surgical approach (i.e. reduction of trauma, reduced blood loss and less infections and also a decrease in intensive care unit and in-hospital stay), that accompany decreased activation of the neurohumeral system and subsequent lower impairment of kidney function (14), we expected a high significance in the mean difference of renal parameters between both surgical approaches.
Full table
The reasons for these contradictions remain unclear, but might be related to the lower number of female patients (male vs. female: n=80:23) and the patients, who underwent less invasive surgery (SAS vs. LIS: n=90:13) in our study, or to potential baseline differences in those groups (e.g., mean age, degree of renal failure or preoperative ejection fraction).
It is likely that a larger study population with equally distributed baseline characteristics may provide a clearer dataset for the differences between both gender and surgical approach should be found, with regard to statistical significance.
Our study therefore confirms the findings of previous studies investigating the impact of ventricular assist devices on kidney function. Moreover, we found a higher improvement of renal parameters after less invasive LVAD implantation in comparison to the standard surgical approach via sternotomy. There was also no significant difference between the mean change of renal parameters between male and female patients. Nevertheless, we expect that in a higher study population with equally distributed baseline characteristics, significant differences in the mean changes of renal parameters may be found.
Further studies on a larger data set are warranted to improve statistical significance of the data and uncover long term results of less invasive LVAD implantation.
Conclusions
LVAD implantation improves kidney function in patients with renal dysfunction. A considerable difference in the change of renal parameters was detected in patients with LIS as compared to SAS, which was not significant possibly due to the limited size of the patient cohort (n=13).
Acknowledgements
None.
Footnote
Conflicts of Interest: JDS is a consultant for Thoratec Corporation as well as HeartWare Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was performed as per the principles outlined in the Declaration of Helsinki and approved by the local institutional review board.
References
- Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol 2013;168:934-45. [Crossref] [PubMed]
- WRITING GROUP MEMBERS, Lloyd-Jones D, Adams RJ, et al. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation 2010;121:e46-215. [Crossref] [PubMed]
- Schwarz C.. Herzinsuffizienz und Niereninsuffizienz. Wiener Klin Mag 2009;12:38-43. [Crossref]
- McCullough PA, Kellum JA, Haase M, et al. Pathophysiology of the Cardiorenal Syndromes: Executive Summary from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:82-98. [Crossref] [PubMed]
- Tromp TR, de Jonge N, Joles JA. Left ventricular assist devices: a kidney’s perspective. Heart Fail Rev 2015;20:519-32. [Crossref] [PubMed]
- Cruz DN, Schmidt-Ott KM, Vescovo G, et al. Pathophysiology of Cardiorenal Syndrome Type 2 in Stable Chronic Heart Failure: Workgroup Statements from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:117-36. [Crossref] [PubMed]
- Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422-30. [Crossref] [PubMed]
- Okabe T, Yakushiji T, Kido T, et al. Relationship between worsening renal function and long-term cardiovascular mortality in heart failure patients. Int J Cardiol 2017;230:47-52. [Crossref] [PubMed]
- Patel AM, Adeseun GA, Ahmed I, et al. Renal Failure in Patients with Left Ventricular Assist Devices. Clin J Am Soc Nephrol 2013;8:484-96. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: Upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally invasive ventricular assist device surgery. Artif Organs 2015;39:473-9. [Crossref] [PubMed]
- Rojas SV, Hanke JS, Avsar M, et al. Left Ventricular Assist Device Therapy for Destination Therapy: Is Less Invasive Surgery a Safe Alternative? Rev Esp Cardiol (Engl Ed) 2018;71:13-7. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (Heartmate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Hanke JS, Rojas S V, Avsar M, Haverich A, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Mao H, Katz N, Kim JC, et al. Implantable Left Ventricular Assist Devices and the Kidney. Blood Purif 2014;37:57-66. [Crossref] [PubMed]
- Mullens W, Abrahams Z, Francis GS, et al. Importance of Venous Congestion for Worsening of Renal Function in Advanced Decompensated Heart Failure. J Am Coll Cardiol 2009;53:589-96. [Crossref] [PubMed]
- Rojas S V, Hanke JS, Haverich A, et al. Chronic ventricular assist device support: surgical innovation. Curr Opin Cardiol 2016;31:308-12. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Letsou GV, Myers TJ, Gregoric ID, et al. Continuous axial-flow left ventricular assist device (Jarvik 2000) maintains kidney and liver perfusion for up to 6 months. Ann Thorac Surg 2003;76:1167-70. [Crossref] [PubMed]
- Schmitto JD, Deniz E, Rojas SV, et al. Minimally Invasive Implantation: The Procedure of Choice! Oper Tech Thorac Cardiovasc Surg 2016;21:65-78. [Crossref]
- Radovancevic B, Vrtovec B, de Kort E, et al. End-organ Function in Patients on Long-term Circulatory Support With Continuous- or Pulsatile-flow Assist Devices. J Heart Lung Transplant 2007;26:815-8. [Crossref] [PubMed]
- Russell SD, Rogers JG, Milano CA, et al. Renal and Hepatic Function Improve in Advanced Heart Failure Patients During Continuous-Flow Support With the HeartMate II Left Ventricular Assist Device. Circulation 2009;120:2352-7. [Crossref] [PubMed]
- Brisco MA, Kimmel SE, Coca SG, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014;7:68-75. [Crossref] [PubMed]
- Herzkrankheiten: Männer erkranken häufiger, Frauen sterben öfter daran. [cited 2017 July 18]. Available online: https://dgk.org/pressemitteilungen/herzkrankheiten-maenner-erkranken-haeufiger-frauen-sterben-oefter-daran/
- He J, Ogden LG, Bazzano LA, et al. Risk Factors for Congestive Heart Failure in US Men and Women. Arch Intern Med 2001;161:996. [Crossref] [PubMed]


