Port Access (Thru-Port System) video-assisted mitral valve surgery
Introduction
Mitral valve disease is ubiquitous, and takes an important toll in terms of resources, morbidity and mortality. Established international estimates reported in 2006 an age and sex-adjusted prevalence of mitral valve regurgitation of 1.7% and of mitral stenosis of 0.1% in North America (adjustments based on distribution of the US 2000 population census) (1,2). Furthermore, since the first mitral valve operation performed in the twentieth century by Elliot Cutler at the Peter Bent Brigham Hospital in 1923, mitral valve surgery has undergone significant advances in this century leading to the popularization of various surgical approaches. A recent breakthrough in this field has been the introduction of minimally invasive port-access mitral valve surgery performed with increasing frequency since its introduction in 1996 at Stanford University by Pompili et al. (3).
Its widespread use in Europe from the beginning of new millennium was predominantly related to the effort of some pioneer centers (4-8).
To date, port-access and video-assisted mitral valve surgery is considered one of the most innovative and sophisticated, but at the same time controversial, approach amongst the wide variety of minimally invasive techniques in cardiac surgery.
A video-assisted or video-guided right mini-thoracotomy approach (4-6 cm), rather than a standard full median sternotomy, allows, a reduction in the length of the incision, a major increase in patient comfort, a lower risk of morbidity and a shorter in-hospital stay, besides the remarkable cosmetic advantages.
“Quenching” the surgical approach from a standardized full sternotomy to a mini-thoracotomy, makes mandatory, in addition to the use of video-assistance, a complete re-building of the set-up of cardiopulmonary bypass (CPB), aortic occlusion and cardioplegic arrest. The idea is to avoid at least the presence of canulas and instruments through a small surgical access. This is the reason why a complete extra-thoracic CPB with catheter-based endo-aortic occlusion and retrograde cardioplegia still remain the optimum solution for an efficient endoscopic mitral valve surgery programme.
The aim of this review is to focus the attention on the “state of the art” of port-access and video-assisted mitral valve surgery appraising the results compared to the traditional approach, describing different technical strategies and analyzing how to avoid and manage its related complications concomitantly elucidating which procedure is associated with the most favorable risk-benefit and cost-benefit profile.
Surgical technique
Minimally invasive mitral valve surgery includes four types of approaches and mini-access set up, each one including a mini-thoracotomy (working port) as main surgical incision; the specific choices are based on anatomical features of the patient, especially in terms of peripheral arteriopathy, size of arterial vessels and surgeon’s individual preference. These options include:
- Type 1—Full extra-thoracic CPB with external trans-thoracic aortic clamping (TTC);
- Type 2—Full extra-thoracic CPB with endo-aortic clamping;
- Type 3—Central arterial cannulation with external TTC;
- Type 4—Central arterial cannulation with endo-aortic clamping.
All these procedures can be supported by video-assistance mainly during the central part (atrial) of the operation. The use of videoscope, mostly of high resolution, full HD or 3D-equipped, is essential in such a limited surgical approach where appropriate inner illumination and difficult exposure may represent an Achilles’ heel of the procedure. Advantages of videoscope during cardiac surgery have been reported in the past even in standard approaches like full sternotomy (9).
Our surgical experience is mostly based on Type 2
This approach is unquestionably not indicated in case of severe peripheral atherosclerotic disease involving iliac or femoral vessels or abdominal aorta (where Type 4 set up is recommended). A previous operation on right lung or pleura is also a manifest contra-indication to the mini-thoracotomy approach. Finally the unfeasibility in using transesophageal echocardiography (for example esophageal varices grade II, etc.) makes proper use of endo-aortic balloon impossible and unsafe.
The surgical technique consists of the preparation of patient in a supine position with slight anterior-rotation of the right chest (30°) aiming at achieving the optimal exposure for the right mini-thoracotomy (Video 1). Besides standard monitoring, bilateral radial arterial cannulation to ensure optimal endo-aortic occlusion, and intermittent single lung ventilation to better visualize through the working port, are undertaken by the anesthesiologist. Superior vena cava (SVC) cannulation, through a percutaneous approach of the jugular vein with Seldinger technique, is also achieved before the operation using a 14 to 18 Fr canula. In addition, when useful, the coronary sinus for retrograde cardioplegia can be cannulated by a retrograde endo-coronary sinus catheter by anesthesiologist.
A full-equipped column for video-assistance, including CO2 delivery system, is prepared in operating room for screen-guided surgery and situated just in front of the main surgeon.
Trans-esophageal echocardiography (TEE) control is utilized to better evaluate the mechanism of mitral regurgitation, to plan the optimal surgical strategy and to assess the final result. In addition, TEE assistance is crucial for coronary sinus and SVC cannulation and endo-aortic occlusion catheter positioning. A proper view from TEE of arterial or venous vessels during catheter or guide-wire introduction is mandatory in order to avoid any risk of injury. The use of TEE becomes indispensable, even more than in standard surgery, at the end of the operation to detect residual air in cardiac chambers; secondly in order to manage proper volume loading of both ventricles; and lastly in identifying any wall motion abnormality.
From the surgical point of view, a skin incision (4-6 cm) is performed in the right infra-mammary fold or just underneath the nipple in case of a male (working port), to reach an optimal exposure through the 4th intercostal space (Figure 1). Surgical incision varies depending on anatomy and patient gender. It has to be always far enough from the right inferior costal edge in order to avoid “conflict” with the diaphragm’s cupola. If necessary, a pledgetted-stitch is placed in the fibrous center of the diaphragm and retracted to pull it down improving the exposure. A soft tissue retractor is placed to spread the ribs and take apart the subcutaneous tissue. A 10-mm port with trocar is placed in the third right intercostals space on the anterior axillary line to position the 30°, 10-mm axial camera. The 30° camera allows visualization of the whole left atrium irrespective of its anatomical location. Axial camera is held throughout the operation with a flexible holder in order to permit fixed viewing in the screen and facilitate change in the position during different stages of surgery (Figure 2). A similar holder is also used on the opposite side of the bed to hold firmly the atrial retractor during the entire procedure. This is an extremely important issue allowing not only a fixed position of the retractor during surgery but also to pull up the entire left atrial roof improving the exposure. More recently, a 120°, 10-mm camera is preferably used (Endocamaleon®; Karl Storz Endoskope) due its better performance in terms of visualization. Despite inner illumination is not always ideal with this camera, by rotating the “eye” of the optic, the visualization of the cavities becomes excellent.
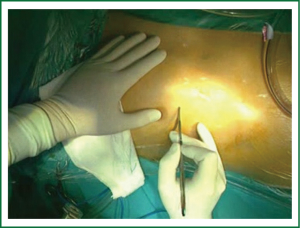
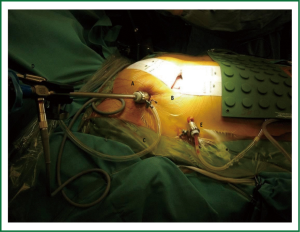
In addition, the 10-mm trocar used in this port allows continuous CO2 insufflation (2-5 L/hour) for de-airing at the end of operation. Trivial CO2 elevation in the blood during CPB can be detected by perfusionist during the delivery, but it can be easily removed during CPB perfusion.
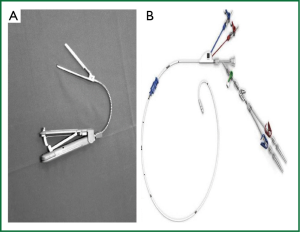
Another 5-mm port is then positioned in the fifth right intercostal space at the median axillary line for the atrial venting canula and the pleural drainage tube at the end of the surgery. At the same time a 2-3 cm incision is performed in the inguinal groove to expose the femoral artery and vein. CPB is preferably established via right femoral vessels. Venous drainage is achieved actively (kinetic or with vacuum) by bi-caval cannulation of both inferior and superior (through the right jugular vein percutaneously) venae cavae. Inferior vena cava is cannulated using Seldinger technique under TEE guidance. By means of a TEE bi-caval atrial view guide-wire and canula are located in proper position with the tip of the inferior cannula just at the entrance of the right atrium. A Y-shaped arterial canula is then introduced in the femoral artery performing a small transverse arteriotomy. When CPB full flow is established, or before to start CPB, a catheter with a balloon at the tip (Endoclamp®; now Intraclude®) is introduced through the side arm of the arterial canula and advanced, under careful TEE guidance, to the ascending aorta 4 cm distal to the aortic valve. The advancement and the proper positioning of the catheter is completed with great precision avoiding injury of the vessels or aortic valve. The guide-wire is firstly advanced and then followed in the arterial tree under TEE-guidance visualizing descending and ascending aorta in short axis and long axis. Only when the guide-wire is properly visualized close to the valve, the catheter is introduced over it to reach the appropriate position in ascending aorta. Recently a new device for endoluminal aortic clamping has been proposed (IntraClude®, Edwards Life Sciences). This system replaces the previous one (Endoclamp®, Edwards Life Sciences) (Figure 3). The IntraClude® device is designed for less traumatic intra-aortic occlusion; its curved shape allows problem-free positioning in ascending aorta making easier the contact with aortic arch and avoiding any possible distal displacement. In addition, a great benefit originates from its reduced size into the lumen of arterial canula, allowing a significant decrease, comparing with previous Endoclamp®, of the pressure rise in the arterial line during perfusion.
After Endoclamp® or IntraClude® insertion, the pericardium is opened in a T-inverted shape, 2 cm anterior to the phrenic nerve. The edges of the pericardium are pulled through the chest using the trocars previously inserted or a needle catheter and hook. This trick allows excellent exposure of the heart pulling the cardiac structures toward the working port.
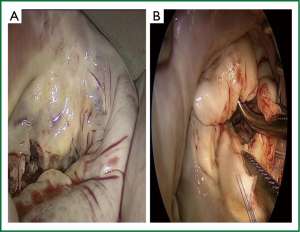
The endoluminal balloon is then inflated with respect to aorta and sino-tubular junction sizes and then antegrade blood cardioplegia is delivered. We usually fill the balloon with saline proportionally to the size of sino-tubular junction and then we check the intra-balloon pressure (around 350 mmHg at the beginning), the TEE view, and the correspondence of both left and right arterial pressure. In absence of complete cardiac activity left atriotomy is performed and an atrial venting system is positioned on the atrial floor. Proper choice of the atrial retractor’s blade is extremely important in order to have an accurate vision of the mitral valve. It is appropriate to use a blade at least 2 cm far from the anterior annulus of the valve and wide enough to avoid the collapse of the atrial wall on viewing of the valve. The valve is then repaired or replaced as required and according to the chosen surgical strategy (10) (Figure 4).
Effectiveness of technique
Over the past two decades, several studies have proved the feasibility, safety and effectiveness of minimally invasive surgery (MIS) in the setting of mitral procedures, even mitral repair for more complex disease (Barlow) (11). Despite longer bypass and sometime cross-clamp times, early short-term results for mitral valve repair using MIS have shown similar outcomes to mitral valve repair performed through standard full sternotomy. Indeed, MIS was associated with a shorter time to extubation even if it usually did not translate into a reduced post-operative in-hospital stay (12). Anyway the absence of evidence does not necessarily translate to evidence of absence. Actually, it remains controversial if the potential benefits of MIS, such as shorter time to extubation, less pain and lower risk of wound infections, could counterpoise the potential disadvantages of longer bypass time and a limited exposure. Grossi et al. reported encouraging short-term results with MIS in degenerative mitral valve disease, in terms of decreased peri-operative morbidity, reduced blood loss, fewer infectious complications and shorter in-hospital stay (13). Holzhey et al. report that MIS is at least as good and safe as the standard full sternotomy approach in elderly patients greater than 70 years with higher peri-operative risk (assessed by Euroscore I logistic =6.0-9.0). They showed 30-day mortality of 7.7% vs. 6.3%; P=0.82 and MACCE 11.2% vs. 12.6%; P=0.86 for minimally invasive and sternotomy patients, respectively. Only the incidence of post-operative arrhythmias and pacemaker implants was significantly higher in the sternotomy group 65.7% vs. 50.3%; P=0.023; 18.9% vs. 10.7%; P=0.059). Also the long-term survival was similar in both groups (66%±5.6% vs. 56%±5.5% at 5 years; and 35%±12% vs. 40%±7.9% at 8 years; P=0.43) revealing that minimally invasive mitral valve surgery is a safe and effective surgical approach, even for elderly patients with moderately higher peri-operative risk (14). Recently, Grossi et al. have validated the long-term efficacy of MIS in terms of freedom from reoperation or recurrent functional and echocardiographic mitral regurgitation, confirming similar outcomes achieved with the standard full sternotomy approach (15). The use of minimal access mitral surgery with endo-aortic occlusion is specifically indicated in redo cases. The presence of a previously implanted prosthetic aortic valve or patent coronary grafts make particularly useful the lateral approach and the use of videoscope. It allows minimal adhesion dissection of intra-pericardial structures, a reduced risk of peri-operative bleeding, short intensive care unit (ICU) and in-hospital stay (16).
The issue whether the short- and long-term good results of minimally invasive mitral valve repair are surgeon-dependent or not has been recently addressed. Holzhey et al. have reported that, a true learning curve really exists for MIS-mitral valve and a substantial number of interventions are required to overcome this learning curve. However remarkable variations exist between individual surgeons (17).
External clamp and endo-aortic clamp
Different studies have been conducted to compare the external TTC with the EBO with controversial results. Both the techniques as expected are seldom accompanied by disadvantages and procedural accidents during the learning curve stage (18,19). While TTC is a relatively cost-effective and apparently easy-to apply method providing the opportunity of establishing CPB via a different access, however it needs conventional antegrade cardioplegia through the aortic root and this can occasionally damage the aortic wall, producing bleeding and even aortic dissection. Such severe complications have been reported during minimally invasive mitral valve surgery, occasionally requiring conversion to full sternotomy. Vollroth et al. reviewed the main reasons and early postoperative outcome for those patients who underwent conversion to full sternotomy. In their series with external TTC the incidence of conversion was only 1%, even if it was associated with a high operative mortality (23.5%). Different surgical sites bleeding was the most common indication for conversion (52.9%; 5 cases on left atrial appendage, 4 on left ventricular apex, 4 on ascending aorta, 5 other sites), followed by severe pulmonary adhesions which hampered the classic working port access and mitral valve exposure (17.6%). Acute aortic dissection type A was identified in 14.7% of cases of conversion, most frequently occurred after removal of aortic clamp or at the time of cannulation (20). Cross clamping an ascending aorta with a trans-thoracic clamp through a small working port is not the same as we usually do through standard sternotomy. Both jaws of the clamp come up from the lateral side of the aorta and some dangerous injuries, including acute aortic dissection as well as bleeding from left atrial appendage and pulmonary artery, have been reported. Consequently, trans-thoracic clamp should be placed very safely and under visual examination of the ascending aorta and the left atrial appendage. In case of technical difficulty, especially in elderly patients with friable tissues, the placement should be aborted in favor of an elective full sternotomy.
EBO is the best established system that enables port-only endoscopic cardiac surgery, without the need for a cardioplegia canula in the ascending aorta, allowing aortic clamping and cardioplegia administration at the same time. Its use is excellent in redo cases, including those cases with patent coronary grafts, without the need to accurately dissect the ascending aorta. Initial reports with Endoclamp® use were unsatisfactory especially for the unusual high incidence of vascular complications (4). This experience was probably related to stiffer catheters during the early practice with this kind of system and also learning curve associated with intra-operative images during positioning (fluoroscopy and early TEE). Today reported incidence of vessel injury (iliac or aortic rupture or dissection) is low (10) and probably related more to the technique of insertion rather than to the catheter itself. Use of intra-aortic occlusion requires tricks and tips to be known. For teams where the use of endo-aortic occlusion is a standard procedure, the incidence today of severe vascular complications is very low. Proper positioning in ascending aorta is crucial not only for adequate aortic occlusion and myocardial protection but also for an optimal exposure of the mitral valve. Although the new Intra-Clude® system seems to be enhanced in this respect, care is necessary to avoid excessive length of the catheter in descending aorta (“slack”) generating an unlikable migration to the valve. Finally, from a technical point of view, endo-aortic clamp is not indicated for patients with dilatation of the ascending aorta (more than 35 mm at sino-tubular junction), peripheral vascular disease and bulky plaques in descending aorta and arch. Similarly, trans-thoracic clamp is unadvisable when pulmonary artery is enlarged and tense or when aorta is suspected to be very tiny and fragile. Few reports have been published, even recently, comparing endo-aortic occlusion and external trans-thoracic clamp during minimally invasive mitral surgery (21). Unfortunately, not many prospective analyses have been reported but usually authors compare the first part of the learning curve when they use endo-aortic occlusion with the second part when they have shifted to external clamp. Hence, clinical results (such as bleeding, conversion rate, duration of operation, etc.) are usually more related to the phase of the learning curve than to the method for occluding the aorta. Recent data suggest that both approaches are safe and comparable, with low risk of morbidity and mortality (no peri-operative death in both groups, 100% 5-year freedom from reoperation in mitral valve repair patients in both groups). Post-operative levels of myocardial cyto-necrosis enzymes as well as ICU times are not significantly different in the two groups. The only one difference was with regards to micro-embolic events which were more frequent with external trans-thoracic clamping rather than intra-aortic occlusion (22). Recent results from Krapf et al. show no differences between TTC and EBO for remote access perfusion (RAP), in terms of peri-operative mortality and morbidity, as well as technical feasibility, procedural success and occurrence of RAP-associated conversions and major complications. Consequently, this study confirms comparable results between the two techniques during RAP and peripheral cannulation (23). Grossi et al. claim that retrograde arterial cannulation via femoral artery instead of central aortic cannulation exposes the patient to an increased risk of stroke in case of older patients with peripheral vascular disease and aortic atherosclerosis (P=0.04; OR 8.5; 95% CI, 1.1-72). This confirms the importance of pre-operative evaluation of the aorta and peripheral vessels with computed tomographic angiography if retrograde arterial perfusion is intended to be planned especially in older patients (24).
Conclusions
Minimally invasive approaches for mitral valve repair and replacement have been reported with increasing frequency over the past 15 years. Different surgical strategies have been adopted as a consequence of individual preferences and historical background. Sometimes dissimilar set-ups have been criticized comparing different stages of learning curve. There is an ongoing discussion regarding the advantages and disadvantages of a minimally invasive approach for mitral valve surgery. Our policy is to make use of the minimally invasive approach, video-assisted right mini-thoracotomy with endo-CPB and EBO whenever possible. This choice is supported by the evidence that, after an initial learning curve, it is a safe and effective approach in terms of short- and long-term results, mainly for redo operations and even for elderly patients with moderately elevated peri-operative risk.
We believe that all these developments applied to minimally invasive procedure, as well as the clinical experience in thousands of patients worldwide have led to a global improvement and to an implementation of this promising and ground-breaking surgical approach. Meanwhile, it is mandatory to persist to devote substantial resources in this field focusing on possible improved tools in order to achieve better risk stratification and prognostication of patients with mitral valve disease undergoing MIS. Although further prospective studies are needed to better determine minimally invasive procedures, we think that the possibility of exploiting and merging clinical and anatomic variables will represent the next major improvement helping in overcoming the current limitations of this approach and providing new insight for a large scale deployment of this technique.
Acknowledgements
Disclosures: Ernesto Greco, MD, PhD has consulted for Edwards Lifesciences on minimally invasive valve surgery.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [PubMed]
- Prifti E, Frati G, Bonacchi M, et al. Accessory mitral valve tissue causing left ventricular outflow tract obstruction: case reports and literature review. J Heart Valve Dis 2001;10:774-8. [PubMed]
- Pompili MF, Stevens JH, Burdon TA, et al. Port-access mitral valve replacement in dogs. J Thorac Cardiovasc Surg 1996;112:1268-74. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [PubMed]
- Viganò M, Minzioni G, Spreafico P, et al. Minimally invasive surgery with the Port-Access method. Preliminary experience. G Ital Cardiol 1998;28:1225-9. [PubMed]
- Ceriana P, Pagnin A, Locatelli A, et al. Monitoring aspects during port-access cardiac surgery. J Cardiovasc Surg (Torino) 2000;41:579-83. [PubMed]
- Greco E, Barriuso C, Castro MA, et al. Port-Access cardiac surgery: from a learning process to the standard. Heart Surg Forum 2002;5:145-9. [PubMed]
- Schroeyers P, Wellens F, De Geest R, et al. Minimally invasive video-assisted mitral valve surgery: our lessons after a 4-year experience. Ann Thorac Surg 2001;72:S1050-4. [PubMed]
- Greco E, Mestres CA, Cartañá R, et al. Video-assisted cardioscopy for removal of primary left ventricular myxoma. Eur J Cardiothorac Surg 1999;16:677-8. [PubMed]
- Greco E, Zaballos JM, Alvarez L, et al. Video-assisted mitral surgery through a micro-access: a safe and reliable reality in the current era. J Heart Valve Dis 2008;17:48-53. [PubMed]
- Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg 2011;142:77-83. [PubMed]
- Suri RM, Schaff HV, Meyer SR, et al. Thoracoscopic versus open mitral valve repair: a propensity score analysis of early outcomes. Ann Thorac Surg 2009;88:1185-90. [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001;71:807-10. [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [PubMed]
- Ricci D, Pellegrini C, Aiello M, et al. Port-access surgery as elective approach for mitral valve operation in re-do procedures. Eur J Cardiothorac Surg 2010;37:920-5. [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- Chan EY, Lumbao DM, Iribarne A, et al. Evolution of cannulation techniques for minimally invasive cardiac surgery: a 10-year journey. Innovations (Phila) 2012;7:9-14. [PubMed]
- Vollroth M, Seeburger J, Garbade J, et al. Minimally invasive mitral valve surgery is a very safe procedure with very low rates of conversion to full sternotomy. Eur J Cardiothorac Surg 2012;42:e13-5; discusson e16.
- Mazine A, Pellerin M, Lebon JS, et al. Minimally Invasive Mitral Valve Surgery: Influence of Aortic Clamping Technique on Early Outcomes. Ann Thorac Surg 2013; [Epub ahead of print]. [PubMed]
- Loforte A, Luzi G, Montalto A, et al. Video-assisted minimally invasive mitral valve surgery: external aortic clamp versus endoclamp techniques. Innovations (Phila) 2010;5:413-8. [PubMed]
- Krapf C, Wohlrab P, Häußinger S, et al. Remote access perfusion for minimally invasive cardiac surgery: to clamp or to inflate? Eur J Cardiothorac Surg 2013;44:898-904. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68-70. [PubMed]