Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis
Introduction
Lung cancer is the leading cause of cancer-related death (26.5% of all) and has the second highest annual incidence in both males and females (13.3% for each), in the United States (1). More than half of lung cancer patients (57%) have distant metastasis at diagnosis, and overall 5-year relative survival rate is as low as 18% (1). Lung cancer can be divided into small cell lung cancer (SCLC, less than 20%) and non-small cell lung cancer (NSCLC, more than 80%), with entirely different treatment strategies and prognosis (2). Treatment strategies mainly include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy (2). Therein, approximately 69% of NSCLC patients with stages I and II are treated by surgery, compared to only 9% of patients with stages III and IV (2). Surgery is considered the best treatment option for early stage lung cancer, and anatomical lobectomy with N1 and at least three N2 stations sampling or dissection has become the standard surgery for early NSCLC patients (3). Minimally invasive video-assisted thoracoscopic lobectomy is also preferred for stage I NSCLC patients (4).
Pneumonectomy and lobectomy have been the main surgery options for lung cancer since the 1940s. In the 1970s, it was reported that limited resection (especially segmentectomy) may be a more reasonable and justifiable option for lung cancer patients (5). Although the complication rate is lower than that of lobectomy, the safety and effectiveness of segmentectomy is still controversial. To date, only one large-scale randomized trial compared the safety and effectiveness of lobectomy and limited resection in T1N0M0 (≤3 cm) NSCLC patients, beginning in 1982 and concluding in 1988 (6). Although a positive impact of limited resection (segmentectomy and wedge resection) on lung function preservation was observed, the authors also showed that patients treated by limited resection had 75% and 30% increase in recurrence rate and overall death rate, respectively (P=0.02; P=0.08) (6). This indicated that segmentectomy should not be the first choice in the treatment of T1N0M0 NSCLC patients. However, in recent years an increasing number of retrospective studies and meta-analyses proposed that limited resection may be superior to lobectomy for early-stage NSCLC patients (7,8). Segmentectomy may be chosen for patients who are intolerant of lobectomy or for palliative purposes (9). Nevertheless, controversy still exists due to the lack of randomized trials and patient selection bias in available studies (10). In the latest National Comprehensive Cancer Network (NCCN) clinical practice guidelines for NSCLC (version 2. 2017) it is suggested that segmentectomy could be selected as treatment, especially for patients with poor lung function or other severe comorbidities and peripheral nodes ≤2 cm (11).
Surgery should not only result in complete resection, but also in a good quality of life. The surgical choice for patients may not be universal, as evidence suggests that senior patients or patients with intolerance to lobectomy may favor segmentectomy. However, segmentectomy typically involves less lymph nodes (LN) dissection than lobectomy, which may explain the higher recurrence observed in patients treated by this method. Recently, a population study indicated that lobectomy harbored a better survival than segmentectomy in NSCLC patients with tumors measuring ≤1 cm or 1 to 2 cm (12). Although several studies have shown a better survival benefit of lobectomy, only few have investigated the impact of extent of lymph node removal on the long-term outcome. Khullar et al. reported that for T1aN0M0 patients, lobectomy may be a good choice, as it was superior to wedge resection or segmentectomy in both overall survival (OS) and 30-day mortality (13). Moreover, this analysis revealed that segmentectomy was frequently accompanied by the examination of ≤3 LN and nodal upstaging. Insufficient and inadequate lymphadenectomy resulted in more false-negative stage I, and, consequently, worse long-term outcomes. Surgical techniques are part of NSCLC staging, and precise staging is essential to aid in the treatment course (14). We hypothesized that the regional extent of lymph node removal may affect patient survival, when treated by lobectomy or segmentectomy. Adequate lymph node management accompanying segmentectomy resulted in comparable oncologic outcome compared to the lobectomy.
Methods
Data source
A retrospective study was conducted by acquiring data from the Surveillance, Epidemiology and End Results (SEER) database (15,16). Data were obtained by SEER*STAT 8.3.2, in October 2016. Enrolled patients met the following inclusion criteria: (I) stage I NSCLC patients; (II) treated via lobectomy (Code 30 or 33) or segmentectomy (Code 22); (III) only one primary tumor; (IV) tumor size ≤3 cm. Exclusion criteria were as follows: (I) surgery to distant site or nodes; (II) unknown scope of regional lymph node surgery or tumor size; (III) unknown or any other TNM (tumor-node-metastasis) stage; (IV) distant or unknown summary stage.
This research was approved by the Ethics Review Committee of the Shandong Provincial Hospital Affiliated to Shandong University (No. 2017-221).
Variables
Extent of lymph node removal was measured by the term ‘Scope of Regional Lymph Node Surgery’ (Scope of Reg. LN Sur.) in the SEER database (17). ‘Scope of Reg. LN Sur.’ referred to the removal, biopsy, or aspiration of regional LN at the time of surgery, and was based on surgical procedure. The term ‘Regional Lymph Node Examined’ (Reg. LN Examined) indirectly reflected the extent of lymph node removal, through the pathological examination of the number of regional LN and reporting in micro description. Additional information on these two terms, as well as inclusion and exclusion criteria, were described in the Supplementary Methods.
Data extracted included demographical, pathological, treatment and follow-up information, such as patient ID, sex, age, race, location, laterality, histology, surgical procedure, tumor size, scope of regional lymph node surgery, regional LN examined, radiation, summary stage, differentiation, survival status, dead cause, and survival time. Summary stage, also called SEER staging, is a term in SEER codes, different from TNM staging, which describes how far the cancer has spread from the origin (further explanation in the Supplementary Methods) (18). Long-term outcomes referred to survival status, OS and lung cancer-specific survival (LCSS). Positive result for OS analysis reflected death of all causes, while LCSS analysis only considered lung cancer-specific deaths.
Data from Shandong Provincial Hospital
We consecutively collected data from Stage I (≤3 cm) patients who underwent sublobectomy or lobectomy at Shandong Provincial Hospital from 2006 to 2011. Follow-up, variables, endpoints, and statistics were described previously (19). Limited to the smaller sample size compared to SEER database, we explored the prognostic difference between lobectomy and sublobectomy, not segmentectomy.
Statistical analysis
Raw variables were all categorical, except for age (at diagnosis), tumor size and regional LN examined. For further analysis, these three variables were transformed into categorical data, according to clinical significance and statistical analysis. The median age of selected patients was 65, which was consistent with conventions (20). Tumor size was stratified into three layers: ≤1 cm, 1 to 2 cm, and 2 to 3 cm, according to the Eighth Edition of the TNM Classification for Lung Cancer from the IASLC Lung Cancer Staging Project (21). Chi-square test was used to investigate the association between two surgical options and other clinical/pathological factors. Survival curves were produced using the Kaplan-Meier method and evaluated by log-rank test. To evaluate the effects on prognosis of both lobectomy and segmentectomy, univariate and multivariate Cox proportional hazards regression was applied, after testing proportional hazards assumption through the Kaplan–Meier survival curves, and results were presented as hazards ratio and 95% confidence interval (HR and 95% CI).
There were far less patients treated by segmentectomy, than by lobectomy (1,156 vs. 17,748). Moreover, there were large imbalances in baseline characteristics, such as age, tumor size, Scope of Reg. LN Sur., and differentiation, which might influence long-term outcomes. Thus, propensity score matching (PSM), evaluated by overall balance test, was used for a more objective comparison (22-24). PSMs were described and adjusted for variables including age, tumor size, Scope of Reg. LN Sur., differentiation, histology and radiation. According to the PSM, patients receiving segmentectomy and lobectomy were matched 1:1, and the caliper was 0.2. Moreover, the matching algorithm was nearest neighbor matching, and the estimation algorithm was logistic regression. After PSM, the p value for the overall balance test was 0.746, meaning that neither covariate, nor the whole model, exhibited a large imbalance. All statistical calculations were performed via SPSS 22.0 (SPSS, Chicago, IL, USA). All P values were 2-tailed, and values of less than 0.05 were considered statistically significant.
Results
Baseline information from the SEER project before and after PSM
After selection, 1,156 stage I NSCLC patients who underwent segmentectomy, and 17,748 who underwent lobectomy, were included in this retrospective cohort study. Obvious differences in sex, age, location, laterality, differentiation, scope of regional LN surgery, size, and radiation, were noted between the two surgical groups (Table 1). Specifically, the segmentectomy group had higher numbers of female patients, elderly patients and left laterality, fewer regional LN removed, smaller tumor size, and adjuvant radiation. This indicated that the baseline characteristics of the two groups were not well-balanced.
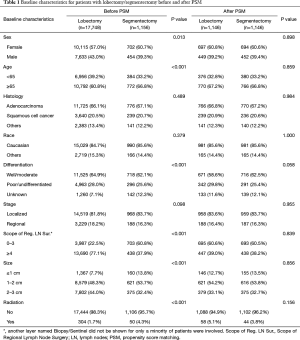
Full table
After the 1:1 PSM, 1,146 stage I patients treated by segmentectomy and 1,146 patients treated by lobectomy were enrolled in the final analysis (10 patients with segmentectomy were not matched and excluded; Table 1). In the final analysis model, baseline characteristics, including sex, age, histology, race, differentiation, LN removed, stage, size and radiation, were all balanced.
Overall prognosis information from the SEER project before and after PSM
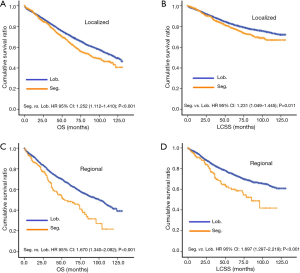
Before PSM, segmentectomy was inferior to lobectomy in both OS [HR: 1.316 (1.186–1.461), P<0.001] and LCSS [HR: 1.310 (1.142–1.504), P<0.001; Figure 1]. The 5-year survival rates of the segmentectomy and lobectomy groups were 61.8% and 70.6%, respectively (P<0.001). Univariate Cox regression analysis indicated that male, elder age, non-adenocarcinoma, poor/undifferentiated, regional stage, segmentectomy, removal of fewer regional LN, larger tumor size, and radiation, were indicative of worse OS and LCSS (Tables S1 and S2). Multivariate Cox analysis indicated that the surgical options were not independent predictors of OS and LCSS (OS: P=0.094; LCSS: P=0.413).

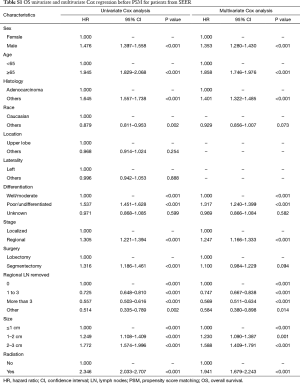
Full table
Full table
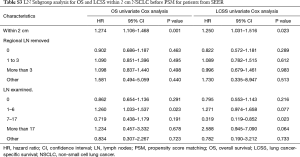
After PSM, OS and LCSS for segmentectomy and lobectomy were similar (Figure 1). A 5-year survival rate of 65.9% was found in the segmentectomy group, and 61.9% in the lobectomy group (P=0.051), and a similar trend was also observed for the 10-year survival rate (42.7% for lobectomy, 40.0% for segmentectomy; P=0.205). Moreover, segmentectomy was no longer a risk factor for OS and LCSS when compared to lobectomy (Tables 2 and 3; OS: P=0.286; LCSS: P=0.692). Interestingly, patients with more regional LN removed had higher OS, as evidenced by the univariate and multivariate Cox regression analyses (compared to no regional LN removed, 1–3: HR =0.731 for univariate and 0.712 for multivariate; more than 3: HR =0.598 for univariate and 0.579 for multivariate; all P<0.001). A similar conclusion could be drawn from the LCSS analysis (regional LN removed 1–3 vs. 0: HR =0.699 for univariate and 0.673 for multivariate; more than 3 vs. 0: HR =0.527 for univariate and 0.506 for multivariate; all P<0.001). Other risk factors, including sex, age, histology, summary stage, differentiation, tumor size, and radiation, still remained independent prognostic factors for both OS and LCSS.
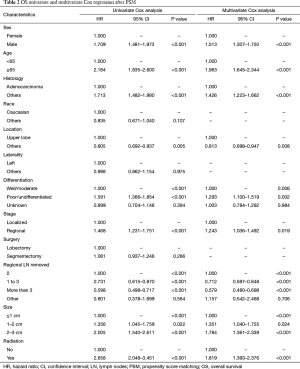
Full table
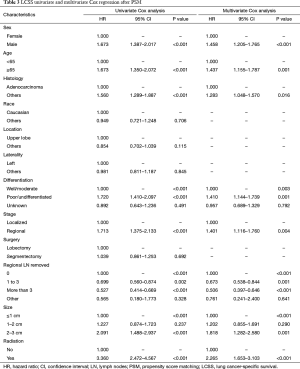
Full table
Prognosis information from the SEER project in each Scope of Reg. LN Sur. layer
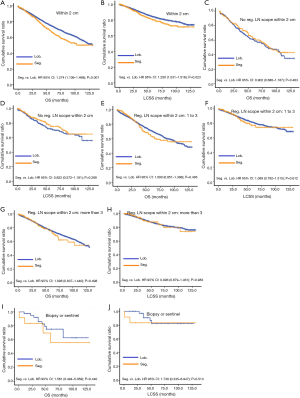
Due to surgical difficulty, segmentectomy significantly favored 3 or less than 3 regional LN removed, whereas lobectomy favored more than 3 (Table 1). If a similar number of LN were removed during surgery, the survival benefit of lobectomy disappeared (Figure 2). Lobectomy did not show any specific survival benefit in each regional LN scope, as follows: (I) no LN removed, OS 0.996 (0.812–1.223), P=0.971 and LCSS 0.988 (0.756–1.292), P=0.931; (II) 1–3 regional LN removed, OS 1.107 (0.924–1.327), P=0.271 and LCSS 1.089 (0.861–1.377), P=0.475; (III) more than 3 regional LN removed, OS 1.116 (0.920–1.352), P=0.266 and LCSS 1.078 (0.832–1.397), P=0.570. However, since the sample size of the biopsy or sentinel groups was small, solid conclusions could not be drawn (lobectomy vs. segmentectomy, 71:15).
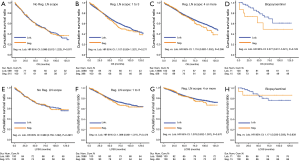
Subgroup analysis for OS and LCSS in SEER project
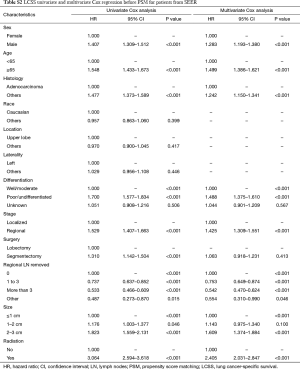
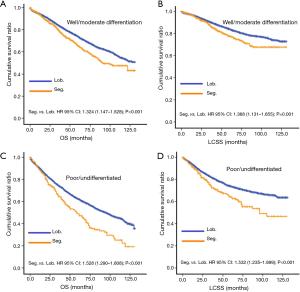
In subgroup analysis before PSM, OS and LCSS of patients who underwent segmentectomy were lower than those of patients in the lobectomy group, for most subgroups, except age <65 years, non-upper lobe, any Scope of Reg. LN Sur. layer, tumor size ≤1 cm, and receiving radiation (Table 4, subgroup analysis: Figure S1-S10). Moreover, regarding solely LCSS, segmentectomy was not significantly inferior to lobectomy in the subgroups ‘squamous cell cancer’ and ‘non-Caucasians’. In the ‘age <65 years’ subgroup, there was no statistically significant difference between the two surgical options in OS and LCSS (OS: P=0.250; LCSS: P=0.572). Interestingly, in the ‘tumor size ≤1 cm’ subgroup, segmentectomy was equivalent to lobectomy in terms of OS and LCSS, independent of regional LN scope (OS: P=0.646; LCSS: P=0.260).
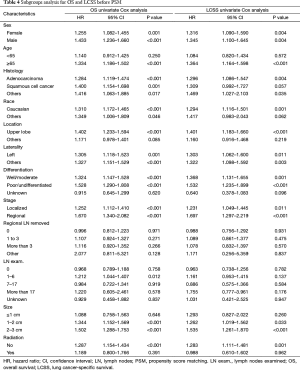
Full table
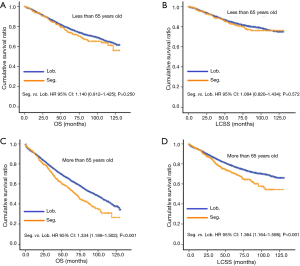
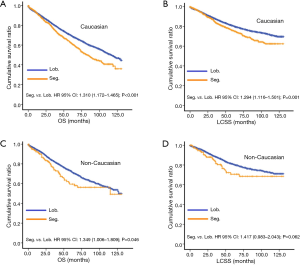
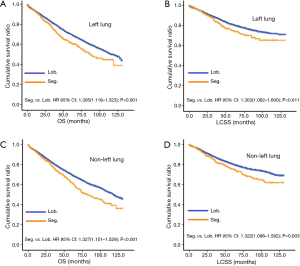
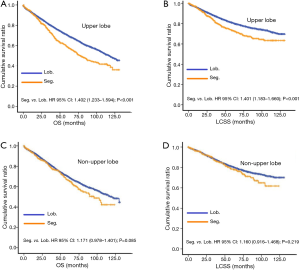
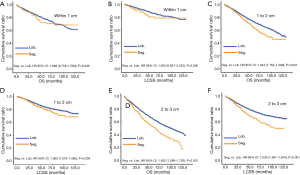
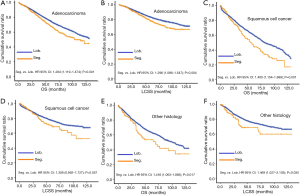

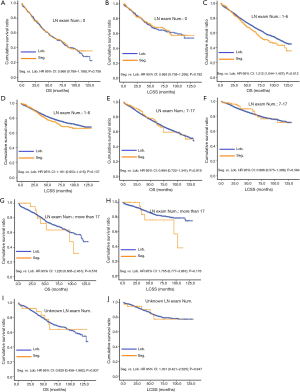
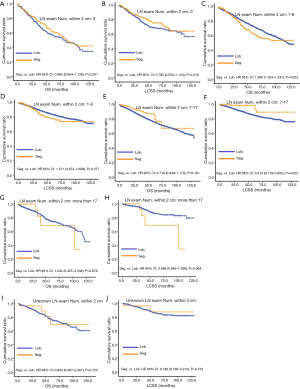
Concerning the number of regional LN examined, segmentectomy was equivalent to lobectomy, regarding both OS and LCSS, in most subgroups, except for OS with 1-6 LN examined (Table 4 and Figure S11). Additionally, we also explored the effect of LN scope or LN examined on OS and LCSS, for stage I NSCLC patients (≤2 cm) treated via either segmentectomy or lobectomy (Figures S12,S13 and Table S3). Overall, segmentectomy was inferior to lobectomy for patients with tumors of ≤2 cm [OS: 1.274 (1.106–1.468), P=0.001; LCSS: 1.250 (1.031–1.516), P=0.023]. However, when balanced with regional LN removed or examined, the survival of patients treated by segmentectomy was similar to lobectomy, except the OS of patients with 1–6 LN examined.
Full table
Validation from Shandong Provincial Hospital
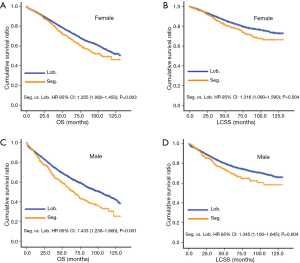
Ultimately, 31 patients underwent sublobectomy and 233 patients underwent lobectomy were included in validation. There was no significant difference between two groups in sex, age, histology, differentiation, tumor size, regional LN removed, and LN examined (Table S4). In overall, there was no significantly prognostic difference between lobectomy group and sublobectomy group (P=0.132, Figure 3). For the reason that baseline characteristics were balanced, we did not perform further PSM analysis. No prognostic difference between two groups in each regional LN removed layer (P in 0, 1–3, and more than 3 regional LN removed: 0.182, 0.590 and 0.203, respectively).
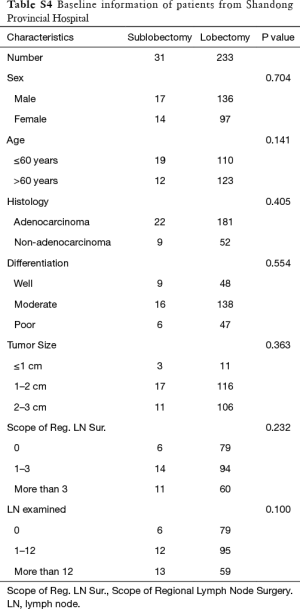
Full table

Discussion
Our retrospective study considered, for the first time, the impact of LN resection on the survival of patients treated via segmentectomy and lobectomy. Obtained results indicated that segmentectomy was equivalent to lobectomy for stage I NSCLC (≤3 cm) patients with analogous regional LN removal. Therefore, segmentectomy could be an alternative to lobectomy in stage I patients, when coupled with sufficient LN dissection or sampling. Furthermore, the removal of a higher number of regional LN predicted better long-term outcomes, which is consistent with our previous research (25).
Our study also indicated that the survival for segmentectomy was similar to lobectomy, especially for T1aN0M0 NSCLC patients, regardless of LN status. That was in agreement with the findings of Kates et al., who observed that for stage I NSCLC patients with tumors of ≤1 cm in size, selected from the SEER database, segmentectomy was comparable with lobectomy in both OS and LCSS (26). Overall, our research revealed that segmentectomy, accompanied by the removal of few LN, may favor senior patients or patients with small tumors. Age, tumor size and the number of LN removed, are all independent predictors of OS. Thus, unbalanced baseline characteristics may result in false-positive conclusions. Since the number of LN examined depends on pathologic reports (different pathologists might report different numbers even when removing the same amount of LN tissue), we focused on the effect of regional LN scope on the prognostic difference between two surgical options (27). Another study indicated that, before PSM, segmentectomy was inferior to lobectomy in 5- and 10-year OS for T1aN0M0 NSCLC patients (tumor size within 2 cm) (28). However, after PSM, there was no longer a difference in OS and recurrence free survival. Our data also showed that these two surgical options might not result in similar survival rates in elderly patients (≥65 years old). This was in accord with Veluswamy’s study, which also pointed out that differences might arise in elderly patients, depending on the different histology subgroups (29). In contrast, both segmentectomy and lobectomy could be optional for younger patients. Cao’s meta-analysis suggested that “intentionally selected” and “compromised” might contribute to the prognostic difference between limited resection and lobectomy (7). Lobectomy might be more adequate for younger patients, whereas segmentectomy should be selected for elderly patients. Interestingly, Razi reported that, for T1aN0M0 NSCLC patients older than 75 years, limited resection could be more appropriate than lobectomy, and an alternative in this high-risk population (30). Another study indicated that segmentectomy was inferior to lobectomy in patients younger than 75 years of age, but not in patients older than 75 years (31). In our study, radiation was related to worse long-term outcomes in T1N0M0 NSCLC patients. This might be because radiation for resected stage I NSCLC was chosen in cases with positive margins or other important risk factors.
For the first time, our study highlighted the importance of the number of LN removed in segmentectomy and lobectomy for stage I NSCLC patients. The reason why more LN scope resulted in a better prognosis might be more accurate staging and less false-negative stage I NSCLC patients (13). Zhou reported that more than 3 LN stations or 10 resected LN were associated with an increase in nodal upstaging and more exact staging (32). Moreover, sampling more than 3 LN stations was found to be an independent predictor for long-term outcomes of lobectomy and segmentectomy in clinical early-stage NSCLC. Mattioli et al. emphasized the application of anatomical segmentectomy in cT1a NSCLC patients (33). Anatomical segmentectomy also involves the dissection of LN stations 4–7 and 10–13. Their study revealed that cancer-specific survival was equivalent between anatomical segmentectomy and lobectomy. Matsumura suggested the reasonable extent of dissection for intentional segmentectomy, for ≤2 cm peripheral NSCLC, included lobar-segmental, hilar and mediastinal LN (34). Moreover, the percentage of limited resection and the number of LN examined increased from 1987 to 2008, for T1aN0M0 NSCLC patients in the SEER database (35). Temporal trends changed and no statistically significant difference in OS between lobectomy and segmentectomy was observed in the late period [2005–2008]. Interestingly, patients with 6–10 LN examined in each period had similar prognosis to those with more than 10 LN examined. This indicates that 10 may not be the discriminative cutoff for the number of LN examined, without unified standard.
There are several limitations in our study. Firstly, it’s a retrospective study. It may have more bias than a prospective study. Secondly, we could not separate “Intentional selected” and “Compromised” from the SEER database. Related information included comorbidity, pre- and post-operative lung function, in-hospital duration and cost was also unavailable. Thus, we could not analyze whether small resection was equal to small operation or less injury (36). Thirdly, the LN removed details were also unavailable to make further detailed analysis.
Conclusions
In summary, for stage I patients, segmentectomy might be also recommendable on the condition of reasonable LN dissection performed. It needs further prospective cohort studies to explore the reasonable surgical options and LN dissection.
Supplementary methods
Scope of regional lymph node surgery
Term Scope of Regional Lymph Node Surgery was a hierarchical variable reflecting the lymph nodes removed by the surgeons at the time of surgery of the primary site or during a separate surgical event. Main options for this term mainly included None (no regional lymph node surgery), Biopsy or aspiration of regional lymph node, NOS, Sentinel lymph node biopsy, 1–3 regional lymph nodes removed (fewer than four lymph nodes), 4 or more regional lymph nodes removed, and Unknown or not applicable. Scope of Regional Lymph Node Surgery in our study referred to: 0; 1 to 3 regional lymph nodes removed; 4 or more regional lymph nodes removed; Biopsy or Sentinel node biopsy.
As Scope of Regional Lymph Node Surgery was presented as a numerical form before 2003 and united into a categorical variable after 2003, we selected the data from January 2003 to December 2013 to reduce information bias.
Regional lymph node examined
Regional Lymph Node Examined was a numerical variable reflecting the number of lymph nodes examined by the pathologists after the surgery. Regional lymph nodes examined was specific or unknown number of lymph nodes examined. The code of this term ranging from 0 to 89 directly meant the concrete number examined. The code 90 meant 90 or more than 90 lymph nodes examined. Other codes meant no, unknown or not applicable number of lymph nodes were examined. Median of the number in this research was 7 after selection. Moreover, Osarogiagbon reported that NSCLC patients in SEER database with 18 to 21 lymph nodes examined had the lowest morality risk (27). Thus, the number of regional lymph nodes examined were divided as 5 groups: 0, 1–6, 7–17, more than 17 and unknown.
Summary stage
Selection of patients in this research was limited to stage I (TNM staging). Here we introduced the summary stage to the prognostic analysis. Summary stage referred to a universal staging method across diverse tumors (details in SEER SUMMARY STAGING MANUAL—2000) and covered 5 options: in situ, localized, regional, distant & unknown (18). In situ meant no evidence of invasion, nodal involvement or metastasis. Localized meant no extension beyond the outer limits of the organ or metastasis. Distant meant distant metastasis. If the carcinoma is not in situ, local or distant, the stage is regional.
In particular, in situ referred to noninvasive lesion or/and intraepithelial lesion for lung cancer. Localized stage referred to lesion was confined to (I) carina; (II) hilus of lung; (III) main bronchus or >2.0 cm from carina; (IV) extension from other parts of the lung to main stem bronchus >2.0 cm from carina; (V) extension from other parts of the lung to main stem bronchus; (VI) single tumor confined to one lung. Regional stage referred to direct extension (atelectasis, obstructive pneumonitis, extension to major blood vessels or nerves, chest wall, main bronchus <2.0 cm from carina, mediastinum, pancoast tumor, pleura, separate tumor nodules in the same lobe or main bronchus), ipsilateral regional lymph nodes involved, both direct extension and ipsilateral regional lymph nodes involved. Distant stage referred to (I) distant lymph nodes involved; (II) extension to abdominal organs, adjacent rib, contralateral lung or main bronchus, heart, pericardial or pleural effusion, skeletal muscle, skin of chest, sternum, vertebra, visceral pericardium; (III) separate tumor nodules in different lobes or contralateral lung; (IV) metastasis. In this research, we merely included in situ/localized (all presented as localized) and regional stage.
Acknowledgements
We thank all patients, medical staff and SEER members relevant to this study.
Funding: This study was funded by Provincial Natural Science Foundation of Shandong (No. ZR2014HM100), National Natural Science Foundation of China (No. 81672288) and Provincial Sci-Tech Development Plans of Shandong (No. 2015GSF118063). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research was approved by the Ethics Review Committee of the Shandong Provincial Hospital Affiliated to Shandong University (No. 2017-221).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Jacobson MJ, Zand L, Fox RT, et al. A comparison of wedge and segmental resection of the lung. Thorax 1976;31:365-8. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [Crossref] [PubMed]
- Jaklitsch MT, Mery CM, Audisio RA. The use of surgery to treat lung cancer in elderly patients. Lancet Oncol 2003;4:463-71. [Crossref] [PubMed]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Oncology NCPGi. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer</= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Vansteenkiste J, Dooms C, De Leyn P. Early stage non-small-cell lung cancer: challenges in staging and adjuvant treatment: evidence-based staging. Ann Oncol 2010;21 Suppl 7:vii189-95. [Crossref] [PubMed]
- SEER. Available online: http://seer.cancer.gov/
- Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3-18. [Crossref] [PubMed]
- SEER Program Coding and Staging Manual 2016. Available online: https://seer.cancer.gov/manuals/2016/SPCSM_2016_maindoc.pdf
- Young JL Jr, Roffers SD, Ries LA, et al. editors. SEER Summary Staging Manual -2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01-4969, Bethesda, MD, 2001.
- Wang K, Qu X, Wang Y, et al. Effect of mu Agonists on Long-Term Survival and Recurrence in Nonsmall Cell Lung Cancer Patients. Medicine (Baltimore) 2015;94:e1333. [Crossref] [PubMed]
- Chan PS, Nallamothu BK, Krumholz HM, et al. Long-Term Outcomes in Elderly Survivors of In-Hospital Cardiac Arrest. N Engl J Med 2013;368:1019-26. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Bertsekas DP, Tseng P. Relaxation methods for minimum cost ordinary and generalized network flow problems. Operations Research 1988;36:93-114. [Crossref]
- Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat 2006;15:609-27. [Crossref]
- Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Statistical Science 2008;23:219-36. [Crossref]
- Yang M, Cao H, Guo X, et al. The number of resected lymph nodes (nLNs) combined with tumor size as a prognostic factor in patients with pathologic N0 and Nx non-small cell lung cancer. PLoS One 2013;8:e73220. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg 2010;90:1651-6. [Crossref] [PubMed]
- Zhou H, Tapias LF, Gaissert HA, et al. Lymph Node Assessment and Impact on Survival in Video-Assisted Thoracoscopic Lobectomy or Segmentectomy. Ann Thorac Surg 2015;100:910-6. [Crossref] [PubMed]
- Mattioli S, Ruffato A, Puma F, et al. Does anatomical segmentectomy allow an adequate lymph node staging for cT1a non-small cell lung cancer? J Thorac Oncol 2011;6:1537-41. [Crossref] [PubMed]
- Matsumura Y, Hishida T, Yoshida J, et al. Reasonable extent of lymph node dissection in intentional segmentectomy for small-sized peripheral non-small-cell lung cancer: from the clinicopathological findings of patients who underwent lobectomy with systematic lymph node dissection. J Thorac Oncol 2012;7:1691-7. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (</= 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Lynch JE, Zwischenberger JB. Is a smaller resection a smaller operation? Chest 2011;139:481-2. [Crossref] [PubMed]


