
Minimally invasive approach for redo mitral valve surgery
Introduction
Redo cardiac surgery represents a clinical challenge due to a higher rate of peri-operative morbidity and mortality (1,2). Mitral valve re-operations can be particularly demanding in patients with patent coronary artery bypass grafts, previous aortic valve replacement, calcified aorta or complications following a previous operation (abscesses, perivalvular leaks, or thrombosis). Risk of graft injuries, hemorrhage, the presence of dense adhesions and complex valve exposure can make redo valve operations challenging through a median sternotomy. Re-sternotomy may also be complicated in patients with vascular structures (brachiocephalic vein, ascending aorta, right ventricle) that lie directly behind the sternum or in patients who had previous sternal wound infections or chest radiotherapy (1-3). In these cases, a minimally invasive surgical approach through a right-sided mini-thoracotomy is a valid alternative to a repeated conventional median sternotomy (4,5). Several technical options have been proposed for minimally invasive mitral reoperations, particularly regarding cannulation sites and type of cannulae for cardiopulmonary bypass (CPB), use of classical aortic clamping, endoclamp or continuous coronary perfusion (beating heart or ventricular fibrillation) with an unclamped aorta as well as the optimal temperature range (5-9). In recent years, percutaneous transcatheter or hybrid procedures with peripheral or alternative thoracic approaches are expanding treatment choices in this selected, often fragile, cohort of patients (10-13).
This review article provides an overview of minimally invasive approaches for redo mitral valve surgery discussing indications, techniques, outcomes, concerns and controversies.
Indications & contraindications
The choice of a minimally invasive approach to perform reoperative mitral surgery is strictly related to surgeon’s preference but its success is dependent on patient selection, personal technical capacities, availability of technological devices, adequate training, and wise cooperation of the staff, including anesthesiologists, perfusionists and nurses (14,15). Clinical and instrumental indications for reoperative valve surgery have been recently published (16) and are data driven and useful, but guidelines need to be adjusted for each individual patient. Minimally invasive mitral reoperations cannot be performed in patients requiring concomitant cardiac procedures other than tricuspid valve operations, atrial fibrillation ablation, or closure of an atrial septal defect or patent foramen ovale. In addition, patients who have previously received a right-sided thoracotomy are excluded because of difficulty mobilizing the lung. Other contraindications are aortic regurgitation >2+ and significant right lung disease with tight pleuro-pericardial adhesions.
The common clinical scenarios for reoperative mitral surgery include patients with native mitral valve structural or functional disease (stenosis, regurgitation or both) after previous non-mitral surgery (coronary artery bypass grafting, aortic valve, aortic root or ascending aorta replacement, congenital septal defects or anomalies) or patients undergoing a second or further procedure on the mitral valve after previous failed repair or replacement (malfunctioning, detachment, thrombosis, degeneration).
With the increasing use of percutaneous trans-catheter procedures for native mitral valve repair (MitraClip), minimally invasive surgical reoperations can become useful options when these innovative strategies fail, as described by Rogers and colleagues in 2009 (17) and by Argenziano and associates in 2010 (18).
Techniques & technology
Minimally invasive mitral valve surgery does not refer to a single approach but rather to a collection of new techniques and operation-specific technologies. These include enhanced visualization and instrumentation system as well as modified perfusion methods, all directed toward minimizing surgical trauma by reducing the incision size (14).
To expose the mitral valve, several skin incisions have been used, including sternotomy, thoracotomy, or percutaneous access. Because of patient demand, marketing forces, and improved technology, the percentage of mitral valve operations done with minimally invasive incisions other than sternotomy have steadily increased to 20% of all mitral operations in 2008 (19,20).
Minimally invasive mitral reoperations are nowadays performed through smaller right thoracotomies termed “mini” thoracotomy or “port access” in the fourth intercostal space, with an incision length somewhere between full thoracotomy (20 cm) and an endoscopic port (0.5 to 1.5 cm) (19). Few are the real alternatives. The New York University group has described a left posterior minithoracotomy approach in 40 patients in whom a right thoracotomy was precluded, e.g., right mastectomy/irradiation (21).
To avoid confusion of terminology, Chitwood et al. (22) proposed a classification system whereby minimally invasive approaches are categorized on the basis of whether the surgeon uses direct vision, thoracoscopic visualization, or robotics for any portion of the surgery (Table 1).

Full table
CPB for minimally invasive mitral reoperations is usually instituted through the cannulation of the femoral artery and the femoral vein (2,3,15,23,24). Alternative sites of arterial cannulation are the ascending aorta (2,4,25,26) or the axillary artery (23,27). Alternative or accessory sites of venous cannulation are venae cavae (bicaval) (2,4) or the jugular vein (2,23).
In patients in whom the aorta can be safely dissected, myocardial protection can be achieved by means of antegrade cardioplegia after classical aortic clamping. Patients in whom the ascending aorta cannot be safely mobilized the use of the endoclamp (routinely used as first choice in several centres) can facilitate surgery. Possible alternatives are the empty beating heart or ventricular fibrillation/fibrillating arrest (spontaneous after systemic cooling or induced with a fibrillator or, more recently, a pacing Swan Ganz catheter) with an unclamped aorta. During beating heart valve surgery, the heart is kept empty and continues to beat, unless systemic temperature induces ventricular fibrillation. In both cases, myocardial protection is achieved through continuous coronary perfusion. The aim of this procedure is to decrease or eliminate the ischemia-reperfusion injury which follows standard manoeuvres of aortic cross clamping and clamp release (28). The chest cavity is usually flooded with carbon dioxide (CO2) to mitigate intracavitary air. Specialized elongated-shaft instruments are used for tissue handling and suturing. De-airing is accomplished with Valsalva manoeuvres and volume filling of the heart, through a trans-mitral vent catheter that exits through the atriotomy and, when feasible, through an aortic root vent.
Our technique
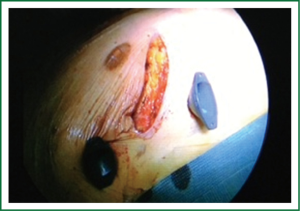
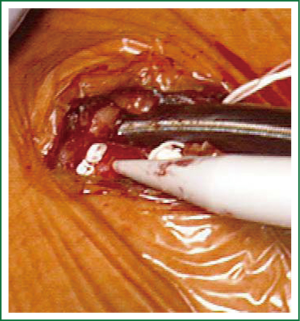
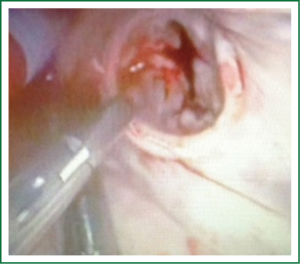
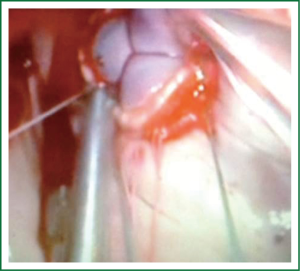
Patients are positioned in a supine position with the right side of the chest slightly elevated. Endotracheal intubation with a double lumen tube and trans-esophageal echocardiogram (TEE) is performed in all patients. External defibrillator pads are applied in all cases. A right antero-lateral thoracotomy is performed through the fourth intercostal space (Figure 1). We prefer the femoral artery for arterial inflow and the femoral vein for venous drainage (Figure 2). Direct aortic cannulation can be used in patients without grafts on the ascending aorta, in case of diffuse aorto-iliac atherosclerosis and in patients with history of multiple femoral accesses (previous operations or angiographic studies). In cases of direct aortic cannulation, a percutaneous femoral venous line is positioned under TEE control. The jugular vein is rarely cannulated to improve venous drainage. With the use of active vacuum on venous drainage, snaring of the venae cave is not always necessary. CO2 is continuously insufflated into the chest throughout the procedure to displace intracardiac air. At the beginning of our experience, patients were cooled to a temperature of 27 or 28 °C and operations were usually performed under fibrillatory arrest. Subsequently, temperature was maintained between 30 and 33 °C to allow operation on the empty beating heart. An aortic vent is always under continuous suction in the ascending aorta to evacuate air. The left atrium is immediately opened in the atrio-ventricular groove. The mitral valve is exposed using an atrial retractor, paying attention to minimize aortic insufficiency to obtain a reasonably bloodless field (Figure 3). An additional left atrial pump sucker is used to maintain a clear operative field. The mitral valve repair or replacement is performed under direct vision (Figure 4 and Video 1). Upon completion of the open heart procedure, ventilation is resumed and air evacuated using an aortic vent and CO2 insufflation. In the event of concomitant mild aortic insufficiency, flows on CPB can be decreased and systemic temperature lowered. If aortic insufficiency is significant, this approach may be contraindicated. When necessary rewarming and cardioversion with external pads is performed and patients weaned off CPB. A drainage tube is placed in the right pleura. Whenever possible, the tip of a second small drain can be introduced in the pericardial space at level of the interatrial groove. Placing temporary pacing wires on right ventricle is often difficult. A possible alternative is through the central venous line. The thoracotomy is closed in a standard fashion.
Outcomes
We reviewed outcomes of minimally invasive approach for redo mitral surgery with a MEDLINE search strategy combining “mitral valve” with the following terms: ‘minimally invasive’, ‘reoperation’, and ‘alternative approach’. The search was limited to the last ten years and additional limits were English language citations and human subjects. In addition, the ‘related articles’ function in PubMed was used as a further check of rigor. Where multiple cohort studies were published by a single institution, the largest or most informative study was included. A total of 168 papers were found using the reported search. From these, ten papers were identified to provide the best evidence on the subject. Principal data of the relevant papers are summarized in Table 2.
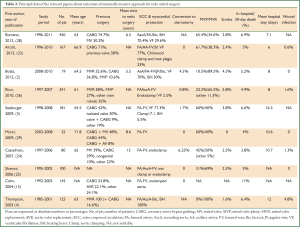
Full table
Although the principal interest in minimally invasive mitral valve approaches has been among patients undergoing elective operations in the primary setting, the avoidance of reoperative sternotomy may represent a more compelling indication for patients with previous cardiac operations (23). In papers reporting outcomes of more than 500 patients undergoing minimally invasive mitral procedures, reoperations represent a variable percentage, from 1% to 35.6% [12 of 1,000 patients, 1%, from 1996 to 2011 in the experience of McClure and colleagues (30); 45 of 1,071 patients, 4.2%, from 1986 to 2008 in the experience of Galloway and colleagues (31); 87 of 789 patients, 11%, from 1999 to 2009 in the experience of Holzhey and colleagues (32); 221 of 1,178 patients, 18.8%, from 1996 to 2008 in the experience of Modi and colleagues (33); and 241 of 677 patients, 35.6%, from 1997 to 2007 in the experience of Ricci and colleagues (26)].
Mean age of patients at reoperation ranged from 61 (26) to 71.8 years (29). Mean time to redo surgery ranged from 5.5 (2) to 15 years (24). Conversion to sternotomy ranged from 0% (23,25,33) to 6.25% (24). Mitral valve repair and replacement ranged from 0% (4) to 65.4% (28) and from 34.6% (28) to 100% (4), respectively. Mortality rates varied but decreased from 11% in the personal experience of Cohn in 2004 (15) to 3% of Arcidi and colleagues in 2012 (23). Stroke rate ranged from 0% of Murzi and colleagues (29) to 5.8% of Ricci and colleagues (26).
Right thoracotomy or mini thoracotomy approach for operation on the mitral valve is not new. It was one of the early approaches to the mitral valve and has been used for more than 60 years. Several previous series have documented its utility and highlighted the advantages in reoperative mitral valve procedures (28).
A right thoracotomy facilitates efficient exposure to the mitral valve with only a moderate retraction. From the right chest, the mitral valve can be easily approached in all cases; the increased distance to the valve can be overcome by the use of longer surgical instruments. In addition, this approach is highly suitable to observe valve pathology and function. Through the same approach it is also possible to reach and control superior and inferior vena cava and to enter the right atrium for additional right heart procedures. Treatment of atrial fibrillation with different devices and different lesion set is also possible, even if it requires more extensive dissection of adhesions (26).
The greatest potential benefit of a right mini-thoracotomy is the avoidance of sternal re-entry and limited dissection of adhesions, avoiding the risk of injury to cardiac structures or patent grafts, and limiting the amount of postoperative bleeding (34). This consistently translates into reduced blood loss, less transfusions and faster recovery. There are several important studies describing a right mini thoracotomy approach for reoperative valve surgery (24,25,35-38) and one describing a left posterior approach (21). The case-control studies all demonstrated superiority of the right mini-thoracotomy versus a reoperative sternotomy. The series from Sharony et al. (25) demonstrated equal mortality (5% for isolated mitral operations), fewer wound infections, less blood product utilization, decreased hospital length of stay, and slightly more favorable mid-term. The important message from this study was that all patients interviewed considered that their recovery was more rapid and less painful than their original sternotomy. Onnasch et al. reported 39 patients undergoing redo mitral valve surgery through a right minithoracotomy with a mortality of 5.1% (38). This group concluded that a minimally invasive approach offers excellent exposure and minimizes the need for mediastinal dissection and optimizes patient comfort.
Concerns & controversies
The limitations to the use of right mini thoracotomy approach are mainly related to a prolonged learning curve that can increase the risk of patients at new centres and to the cost of the devices. Embolism of air remains a concern when left cardiac cavities are opened. Careful de-airing, by means of aortic and left atrial vents, removed only after disappearance of echocardiographic evidence of air bubbles, along with gentle external squeezing of the heart, can reduce this risk. Moreover, the operating field can be continuously flooded with CO2 to mitigate intracavitary air.
The optimal myocardial protection in minimally invasive mitral reoperations still remains controversial. In patients in whom the aorta can be safely dissected, myocardial protection can be achieved by means of antegrade cardioplegia after classical aortic clamping or by means of aortic endoclamping (39). When the ascending aorta cannot be safely mobilized or in case of reduced experience with the use of endoclamp, possible alternatives are performing the redo operation with an unclamped aorta on the empty beating heart or ventricular fibrillation/fibrillating arrest. These procedures have shown good results also in patients with poor ejection fraction or in cases of multiple valve involvement (2,28). Nevertheless, they can be complicated by air embolism, because standard de-airing manoeuvres cannot be performed. In this setting, continuous CO2 insufflation and ascending aorta suction play a key role. Cerebral monitoring with near infrared spectroscopy and trans-cranial Doppler could be of help to monitor embolism and, if not available, moderate hypothermia rather than normothermia should be preferred. In addition, normothermia reduces organ protection from hypothetical adverse events and requires full pump flow which can increase back bleeding from the aorta, interfering with surgical view and compromising the outcome in case of valve repair. Being able to manage a reoperation without aortic clamping can also be useful in cases of endoclamp malfunctioning as well as in cases of incomplete aortic clamping due to dense adhesion between the ascending aorta and pulmonary artery. This issue has been highlighted and addressed by Romano and colleagues who recently published the outcomes of 450 patients that underwent redo mitral valve surgery via a right thoracotomy from 1996 to 2011 at the University of Michigan (28). Of these, 134 patients underwent redo mitral valve surgery with ventricular fibrillation (core temperature 26 °C), and 316 patients underwent beating heart surgery (core temperature 32 °C). These authors concluded that redo right thoracotomy mitral valve surgery on the beating heart is associated with shorter bypass time, less transfusion requirements, shorter postoperative ventilation, and lower mortality. A possible explanation is that the beating heart approach obviates the need for deeper hypothermia and limits subendocardial hypoperfusion mismatches, which are commonly seen with ventricular fibrillation. During electrically induced fibrillation, oxygen delivery to the left ventricle is markedly reduced and coronary flow is redistributed away from the subendocardium. By keeping the heart in its natural beating state with antegrade coronary flow, the risk of reperfusion injury is potentially mitigated. Also during ischemic arrest, myocardial edema increases in the static diastolic state and may cause cardiac dysfunction. By keeping the heart beating, myocardial edema is decreased and function may be maintained, which may be of particular importance in these patients with already impaired ventricular function.
Emerging & future developments
With increasing numbers of patients undergoing bioprosthetic mitral valve replacement, the numbers of elderly patients requiring redo mitral surgery for bioprosthetic dysfunction is growing. From the first successful case of mitral transcatheter valve-in-valve (MTVIV) implantation into a failed mitral bioprosthesis being reported in 2009 (40), percutaneous transcatheter or hybrid procedures with peripheral or alternative thoracic approaches are expanding the treatment choices in these selected, high risk patients (10-13). These therapies are being developed and will require further improvement before wide spread clinical application. MTVIV implantation is usually performed through a transapical approach with a left anterior minithoracotomy in the fifth or sixth intercostal space. Left ventricular apex provides the most direct, shortest and coaxial access to the mitral valve. Antegrade MTVIV implantation has also been reported with success (7,8). Antegrade access to the mitral prosthesis is obtained through either a direct transatrial approach as reported by our group in Milan or via the femoral vein, trans-septally into the left atrium.
In a recent review about MTVIV procedures (11), Cheung and colleagues reported a mortality between 0 and 33% (overall: 30 days mortality 7.5%, late mortality 10%), low transvalvular gradients and the absence of paravalvular regurgitation, concluding that the MTVIV can be considered a well-tolerated alternative to open procedures in patients deemed inoperable or high-risk surgical candidates for redo mitral valve surgery.
In addition, percutaneous techniques are being used to treat mitral paravalvular regurgitation (PVR), which may be related to calcification, infection or tissue friability, and occurs in 5% to 17% of surgical implanted heart valves (10). However, feasibility for percutaneous closure has to be assessed by defining the shape, size and location of the defect. Echocardiography with three-dimensional defect reconstruction is a cornerstone for guiding percutaneous PVR closure. Access to mitral PVR can be either retrograde from the aorta, transvenous transseptal or transapical. In their review (10), Binder and Webb conclude that percutaneous closure of PVR can be an effective and lower risk alternative to reoperation. Meticulous planning and prudent procedural execution by experienced operators ensuring no impingement of the prosthetic leaflets leads to a high success rate of percutaneous PVR repair. The reported risk for emergent surgery and for death as a complication of percutaneous PVR repair in this paper is 1-2%.
Nevertheless, if these innovative less invasive alternatives to open surgery fail, as referred by Rogers and colleagues in 2009 (17) and by Argenziano and colleagues in 2010 (18), minimally invasive surgical reoperations become the only useful option both for patients and for physicians.
Conclusions
Mitral valve re-operations can be safely and effectively performed through a smaller right thoracotomy in the fourth intercostal space termed “mini” thoracotomy or “port access” approach.
The greatest potential benefit of a right mini thoracotomy is the avoidance of sternal re-entry and limited dissection of adhesions, avoiding the risk of injury to cardiac structures or patent grafts.
Good percentages of valve repair can be achieved. Mortality is low as well as major complications.
Minimally invasive procedures with an unclamped aorta have the potential to combine the benefits of minimally invasive access and continuous myocardial perfusion.
Less invasive trans-catheter techniques could be considered as the natural future evolution of structural heart disease and mitral reoperations. The safety and efficacy of these procedures has never been compared to open reoperations in a randomized trial, although published case series and comparisons to historical cohorts suggest that they are an effective and feasible alternative. Ongoing follow-up on current series will further define these procedures and provide valuable clinical outcome data.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Morales D, Williams E, John R. Is resternotomy in cardiac surgery still a problem? Interact Cardiovasc Thorac Surg 2010;11:277-86. [PubMed]
- Botta L, Cannata A, Fratto P, et al. The role of the minimally invasive beating heart technique in reoperative valve surgery. J Card Surg 2012;27:24-8. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann Thorac Surg 2009;87:709-14. [PubMed]
- Thompson MJ, Behranwala A, Campanella C, et al. Immediate and long-term results of mitral prosthetic replacement using a right thoracotomy beating heart technique. Eur J Cardiothorac Surg 2003;24:47-51; discussion 51. [PubMed]
- Salhiyyah K, Taggart D. Beating-heart valve surgery: A systematic review. Asian Cardiovasc Thorac Ann 2009;17:650-8. [PubMed]
- Wang J, Liu H, Xiang B, et al. Keeping the heart empty and beating improves preservation of hypertrophied hearts for valve surgery. J Thorac Cardiovasc Surg 2006;132:1314-20. [PubMed]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol 2010;106:360-8. [PubMed]
- Wang J, Liu H, Salerno TA, et al. Does normothermic normokalemic simultaneous antegrade/retrograde perfusion improve myocardial oxygenation and energy metabolism for hypertrophied hearts? Ann Thorac Surg 2007;83:1751-8. [PubMed]
- Botta L, Cannata A, Bruschi G, et al. Beating heart mitral valve surgery: innovation or back to the past? J Card Surg 2010;25:318-author reply 318-9. [PubMed]
- Binder RK, Webb JG. Percutaneous mitral and aortic paravalvular leak repair: indications, current application, and future directions. Curr Cardiol Rep 2013;15:342. [PubMed]
- Cheung A, Al-Lawati A. Transcatheter mitral valve-in-valve implantation: current experience and review of literature. Curr Opin Cardiol 2013;28:181-6. [PubMed]
- Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol 2013;61:1759-66. [PubMed]
- Bruschi G, Barosi A, Colombo P, et al. Direct transatrial transcatheter SAPIEN valve implantation through right minithoracotomy in a degenerated mitral bioprosthetic valve. Ann Thorac Surg 2012;93:1708-10. [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [PubMed]
- Cohn LH. Evolution of redo cardiac surgery: review of personal experience. J Card Surg 2004;19:320-4. [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [PubMed]
- Rogers JH, Yeo KK, Carroll JD, et al. Late surgical mitral valve repair after percutaneous repair with the MitraClip system. J Card Surg 2009;24:677-81. [PubMed]
- Argenziano M, Skipper E, Heimansohn D, et al. Surgical revision after percutaneous mitral repair with the MitraClip device. Ann Thorac Surg 2010;89:72-80; discussion p 80.
- Glower DD. Surgical approaches to mitral regurgitation. J Am Coll Cardiol 2012;60:1315-22. [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10.
- Saunders PC, Grossi EA, Sharony R, et al. Minimally invasive technology for mitral valve surgery via left thoracotomy: experience with forty cases. J Thorac Cardiovasc Surg 2004;127:1026-31; discussion 1031-2. [PubMed]
- Chitwood WR, Rodriguez E. Minimally invasive and robotic mitral valve surgery. In: Cohn LH. eds. Cardiac Surgery in the Adult . 3rd edition. New York, NY: McGraw-Hill, 2008:1079-1100.
- Arcidi JM Jr, Rodriguez E, Elbeery JR, et al. Fifteen-year experience with minimally invasive approach for reoperations involving the mitral valve. J Thorac Cardiovasc Surg 2012;143:1062-8. [PubMed]
- Casselman FP, La Meir M, Jeanmart H, et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation 2007;116:I270-5. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive reoperative isolated valve surgery: early and mid-term results. J Card Surg 2006;21:240-4. [PubMed]
- Ricci D, Pellegrini C, Aiello M, et al. Port-access surgery as elective approach for mitral valve operation in re-do procedures. Eur J Cardiothorac Surg 2010;37:920-5. [PubMed]
- Totaro P, Zattera G, Alloni A, et al. The axillary artery as an alternative site of cannulation for redo port access-assisted minimally invasive mitral valve surgery: early report of 2 cases. Perfusion 2009;24:357-9. [PubMed]
- Romano MA, Haft JW, Pagani FD, et al. Beating heart surgery via right thoracotomy for reoperative mitral valve surgery: a safe and effective operative alternative. J Thorac Cardiovasc Surg 2012;144:334-9. [PubMed]
- Murzi M, Kallushi E, Tiwari KK, et al. Minimally invasive mitral valve surgery through right thoracotomy in patients with patent coronary artery bypass grafts. Interact Cardiovasc Thorac Surg 2009;9:29-32. [PubMed]
- McClure RS, Athanasopoulos LV, Mcgurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [PubMed]
- Byrne JG, Karavas AN, Adams DH, et al. The preferred approach for mitral valve surgery after CABG: right thoracotomy, hypothermia and avoidance of LIMA-LAD graft. J Heart Valve Dis 2001;10:584-90. [PubMed]
- Bolotin G, Kypson AP, Reade CC, et al. Should a video-assisted mini-thoracotomy be the approach of choice for reoperative mitral valve surgery? J Heart Valve Dis 2004;13:155-8; discussion 158. [PubMed]
- Burfeind WR, Glower DD, Davis RD, et al. Mitral surgery after prior cardiac operation:port-access versus sternotomy or thoracotomy. Ann Thorac Surg 2002;74:S1323-5. [PubMed]
- Walther T, Falk V, Mohr FW. Minimally invasive surgery for valve disease. Curr Probl Cardiol 2006;31:399-437. [PubMed]
- Onnasch JF, Schneider F, Falk V, et al. Minimally invasive approach for redo mitral valve surgery: a true benefit for the patient. J Card Surg 2002;17:14-9. [PubMed]
- Glower DD, Desai B. Transaortic endoclamp for mitral valve operation through right minithoracotomy in 369 patients. Innovations (Phila) 2010;5:394-9. [PubMed]
- Cheung A, Webb JG, Wong DR, et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg 2009;87:e18-20. [PubMed]


