NUT midline carcinoma as a primary lung tumor: a case report
Introduction
NUT (nuclear protein in testis) midline carcinoma (NMC) is a highly aggressive carcinoma arising from midline structures, such as the upper aerodigestive tract and mediastinum. Less than one hundred cases have been reported worldwide until now (1,2). It is refractory to conventional treatments, such as chemotherapy and radiotherapy. The prognosis was reported to be associated with a median survival of 6.7 months and a 2-year overall survival of 19% (2). The largest series of primary pulmonary NMC was reported by Sholl. All the nine cases were detected having intrathoracic or distant metastases and showed a poorer prognosis with a median survival of 2.2 months (3). The one involving the lung and having the chance to be totally resected was exceptionally rare. No data on this entity is available.
Case presentation
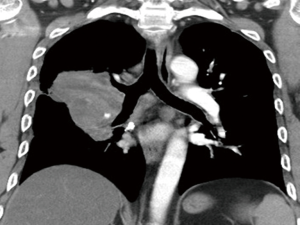
A 48-year-old man with a smoking history of 60 pack years complained of bloody sputum for 1 month and shortness of breath for 1 week. Fever and weight loss hadn’t been noticed. Physical examination showed no evidence of supraclavicular lymphadenopathy or finger clubbing. Chest computed tomography (CT) revealed a perihilar soft tissue mass measuring 7.7 cm × 7.4 cm × 6.0 cm in the right lung, encasing the pulmonary artery trunk and main bronchi, with multiple enlarged lymph nodes (Figure 1). Abdominal ultrasonography demonstrated neither hepatic nor adrenal metastases. 99mTc bone scan and brain magnetic resonance imaging (MRI) were negative. Transbronchial biopsy was routinely performed, but no malignant tumor cell was found in biopsy tissue. He subsequently underwent a video-assisted mini-thoracotomy pneumonectomy of right lung and systematic mediastinal lymphadenectomy with curative intent. Fortunately, the surgery turned out to be a gross total resection.
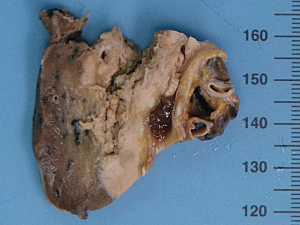
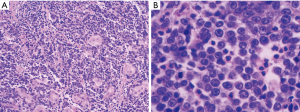
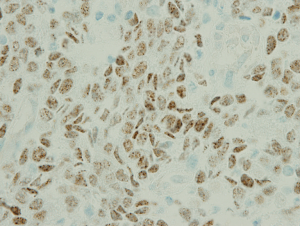
The examination of the specimen revealed a tumor presented with a brownish-whitish section localized within the lung (Figure 2). On permanent histology, sheets of undifferentiated cells with focal squamous differentiation were diagnosed (Figure 3A,B), which led to the possibility of NUT midline carcinoma. Angiolymphatic invasion and perineurial invasion could be found. Surgical endings of hilar vessel and bronchial stump were negative. Lymph nodes were extensively involved: intrapulmonary nodes (3/8), ipsilateral mediastinal nodes (2/9), subcarinal lymph nodes (1/7). A battery of immunohistochemical (IHC) markers were applied. Nearly all the makers expressed negative except P63: P63(++), CD34(−), CK5/6(focal+), TTF-1(−), P40(−), CD117(−), CD5(−), Napsin A(−), Syn(−), CgA(−), CD56(−). P63 was the only positive one which supported squamous cell carcinoma. But the morphological feature excluded this diagnoses. IHC staining with NUT protein revealed diffusely positive (Figure 4). As the anti-NUT C52 monoclonal antibody had a specificity of 100% compared to fluorescent in situ hybridization (FISH), the diagnosis of NMC was confirmed (4). This work was performed with the help of International Nut Midline Carcinoma Registry at the Brigham and Women’s Hospital.
No additional treatment such as chemotherapy or radiotherapy was added following the surgery. He was advised to undergo a routine follow-up. Two months later, he came back to the hospital because of shoulder pain. Palliative radiotherapy to the right scapula and oral analgesics were administered for bone metastatic pain. He passed away 6 months after diagnosis.
Discussion
NMC is a malignant epithelial tumor characterized by chromosomal rearrangements which involve the gene encoding the nuclear protein of the testis on 15q14 (5). It’s an aggressive cancer of squamous cell lineage arising in midline structures, such as head, neck and mediastinum. The first two cases of NMC were independently reported by Kees and Kubonishi in 1991. Both of them were considered originating from thymus (6,7). Since then, cases originating from different organ sites have been reported and explored in depth (1,2,8-11). This unique tumor characteristic of a relatively consistent chromosomal rearrangement is not defined by the site of origin but rather defined genetically.
As a rare tumor, its true incidence remains unknown. It involves patients of all age groups, ranging from several months to 78 years old, but more frequently young patients (2,12-14). It is often unresectable because of high propensity to develop distant metastases when diagnosed. Most patients do not have the chance of a surgery (12). NMC has a poor clinical course with a mean survival of approximate 9 months (15). A series of primary pulmonary NMC was reported in 2015. Through a period of 4 years, nine cases were diagnosed by a NUT IHC screen. The median age was 30 and the most common clinical manifestation was cough. Nearly all cases presented with extensive involvement of mediastinal lymph nodes, pleural disease, intrathoracic or distant metastases. One of them underwent an extrapleural pneumonectomy followed by chemotherapy but died 2 months later. Seven patients received chemotherapy. The median overall survival was 2.2 months (3).
The expression of NUT protein in normal adult is restricted to the testis and ovary. Its function remains unknown. The cytogenetic abnormality of NMC is due to a translocation of the NUT gene with one of the BET family members, about two-thirds were BRD4 on chromosome 19p13.1[t(Jeny15;Jeny19)(q14;p13.1)], rarely BRD3 on chromosome 9q34.2 [t(Jeny15;Jeny9)(q14;p34.2)] (5), or uncharacterized non-BRD genes, such as NSD3 (16). This results in a fusion oncogene as BRD4-NUT, BRD3-NUT or NUT-variant. The fusion proteins are known to bind transcriptionally active chromatin (15), which is thought to block squamous differentiation (17).
It was once included in the category of “carcinoma with t(Jeny15;Jeny19) translocation” by the 2004 WHO Classification of Tumours. As the discovery of alternative fusion patterns by FISH assay, the new edition classified it as “NUT carcinoma” in 2015 (18).
The histological diagnosis of NMC is usually undifferentiated or squamous cell carcinoma (19). Its cytologic features usually present as highly cellular smears, noncohesive with prominent single cells, scant and delicate cytoplasm, variably prominent nucleoli, vesicular chromatin and identifiable mitotic figuresJeny (13). Before the C52 monoclonal antibody to NUT protein developed in 2009, FISH or reverse transcriptase PCR (RT-PCR) was widely used for diagnosis. The NUT-specific C52 monoclonal antibody has a sensitivity of 87%, a specificity of 100%, a negative predictive value of 99% and a positive predictive value of 100% compared to FISH, which was known as the “gold standard” (4). Since its widespread application in IHC, the diagnosis of NMC has been more expedient. However, further laboratory test, such as FISH or next-generation sequencing, is still needed for the detection of concrete fusion pattern.
There is no standard treatment for NMC until now. Patients usually present with metastases at the time of diagnosis. As a consequence, the majority of cases were reported receiving chemotherapy and/or radiotherapy. Only a small part underwent a tumor resection. The histone deacetylase inhibitors (HDACi) had been reported effective to NUT carcinoma. A 10-year-old boy diagnosed of NMC had an encouraging response to Vorinostat (an HDACi). Unfortunately, after stopping the drug because of toxicities, the tumor showed a rapid progression. He died with an overall survival of 11 months. Since then, HDACi has become a focus of research in treating NMC (20). It suggests that NMC may be amenable to therapy designed to induce differentiation. The clinical effect of a bromodomain inhibitor OTX015/MK-8628 targeting BRD4-NUT oncoprotein was reported last year. Four patients with BRD4-NUT fusions were treated with OTX015/MK-8628. Two of them had a rapid response with tumor regression and symptomatic relief. The third one had a relative tumor stabilization. The promising effect hopefully improved the survival of NMC patients (21).
In summary, the present case helps us realize that NMC is a malignancy characteristic of high aggressiveness. It is associated with a poor prognosis even after being totally resected. More effective adjuvant treatments are needed following the surgery. Some targeted therapies have offered strong potential for clinical application. Increasing understanding about the molecular mechanism will guide us to develop more effective treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol 2012;7:247-65. [Crossref] [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Sholl LM, Nishino M, Pokharel S, et al. Primary Pulmonary NUT Midline Carcinoma: Clinical, Radiographic, and Pathologic Characterizations. J Thorac Oncol 2015;10:951-9. [Crossref] [PubMed]
- Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984-91. [Crossref] [PubMed]
- French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 2008;27:2237-42. [Crossref] [PubMed]
- Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19). Am J Pediatr Hematol Oncol 1991;13:459-64. [Crossref] [PubMed]
- Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res 1991;51:3327-8. [PubMed]
- Dang TP, Gazdar AF, Virmani AK, et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst 2000;92:1355-7. [Crossref] [PubMed]
- Vargas SO, French CA, Faul PN, et al. Upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer 2001;92:1195-203. [Crossref] [PubMed]
- Watanabe S, Hirano S, Mine S, et al. A case of endobronchial NUT midline carcinoma with intraluminal growth. Anticancer Res 2015;35:1607-12. [PubMed]
- Klijanienko J, Le Tourneau C, Rodriguez J, et al. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: Report of two new cases and review of the literature. Diagn Cytopathol 2016;44:753-6. [Crossref] [PubMed]
- Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol 2011;5:31-5. [Crossref] [PubMed]
- Bellizzi AM, Bruzzi C, French CA, et al. The cytologic features of NUT midline carcinoma. Cancer 2009;117:508-15. [PubMed]
- French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 2004;22:4135-9. [Crossref] [PubMed]
- French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol 2010;63:492-6. [Crossref] [PubMed]
- French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 2014;4:928-41. [Crossref] [PubMed]
- Grayson AR, Walsh EM, Cameron MJ, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene 2014;33:1736-42. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol 2008;32:828-34. [Crossref] [PubMed]
- Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 2011;71:2686-96. [Crossref] [PubMed]
- Stathis A, Zucca E, Bekradda M, et al. Clinical Response of Carcinomas Harboring the BRD4-NUT Oncoprotein to the Targeted Bromodomain Inhibitor OTX015/MK-8628. Cancer Discov 2016;6:492-500. [Crossref] [PubMed]


