Characteristics of coronary microcirculatory function in patients with Takotsubo syndrome
Introduction
Takotsubo syndrome (TS) is an infrequent syndrome characterized by systolic dysfunction that usually gives a circumferential involvement of the basal and apical segments of the left ventricle (1-3). It is frequently related to acute physical and psychological stress and it is generally reversible (1). From a clinical standpoint, it strongly mimics an acute coronary syndrome (chest pain at rest and/or dyspnea), however coronary angiography reveals the absence of obstructive coronary artery disease or acute plaque rupture (1,3-5). The syndrome has found its own clinical and diagnostic identity only in recent years and it was included by the American Heart Association (AHA) in the classification of cardiomyopathies, as acquired cardiomyopathy (2). More recently the position paper of the ESC suggested using the term “syndrome” instead of cardiomyopathy because the full recovery of patients and the low rate of major adverse cardiac events at follow-up in reported series strongly suggest that TS is different from the primary cardiomyopathies (6).
It was first described in the early 90s in Japan and this had initially suggested that racial and genetic factors could be involved (7). Thereafter, it has been reported with increasing incidence in non-Asian populations, including the United States (8) and Europe (9-10). The term ‘Takotsubo’ derives from the name of the Japanese trap used by fishermen to catch octopuses. This resembles the shape taken by the left ventricle, due to typical wall motion abnormalities involving the apex and the mid segments (1). For this evidence, the syndrome is also called: ‘left ventricular apical ballooning’. The pathogenesis of TS is still uncertain. The proposed hypothesis in the literature is an epicardial multivessel coronary spasm, neurogenic myocardial stunning, catecholaminergic cardiotoxicity, and microvascular dysfunction, being the last two the most accredited. The aim of our study was to investigate the characteristics of coronary microcirculatory function in patients with TS, analyzed by TIMI frame count (TFC), in comparison with patients with microvascular angina (MA) and controls.
Methods
In this multicenter study, we retrospectively selected patients with a diagnosis of TS and we have compared them with a group of control subjects without cardiovascular risk factors, coronary artery epicardial disease and microvascular dysfunction at coronary angiography and with a group of patients suffering from MA. The diagnosis of TS has been made in agreement with the criteria proposed by the Mayo Clinic group (11).
Patients without coronary artery disease were recruited among patients undergoing coronary angiography before scheduled non-coronary cardiac surgery.
The diagnosis of MA was made up if patients had chest pain, ECG changes during the stress test and coronary arteries free from significant stenosis, but a demonstration of microvascular dysfunction at the coronary angiography. Patients with MA were matched by sex and age with TS.
All patients at admission were subjected to careful anamnestic evaluation and cardiovascular risk factors (family history, smoking activity, hypertension, dyslipidemia, diabetes mellitus type 1 and 2, obesity) and history of cardiovascular and cerebrovascular diseases (previous angina, acute myocardial infarction, PCI or stroke/TIA) were recorded. A venous blood sample was collected, on admission, for the evaluation of routine biochemical parameters and the determination of cardiac I troponin (Vitros, Ortho-Clinical Diagnostics). Moreover, patients underwent 12 lead electrocardiogram, transthoracic echocardiogram, and coronary angiography. Left ventricular function was assessed at admission and before discharge by measuring the left ventricular ejection fraction (LVEF), using the biplane Simpson’s method. Coronary angiography was performed by selective catheterization, according to Seldinger’s technique, cannulating a peripheral artery (femoral or radial). Microcirculation was tested by analyzing the TFC according to the Gibson’s technique, as the number of frames required by the contrast medium to reach a standard reference point of a distal coronary vessel (12).
Due to the longer length of LAD, compared to circumflex and right coronary artery, the TFC values were divided by the conventional correction factor for LAD: 1.7 (12).
The TFC in the population of patients with TS was compared with that of controls and with patients with MA. The extent of the alteration and the influence of cardiovascular risk factors, in the genesis of microcirculatory alterations was investigated.
Statistical analyses were performed using the MedCalc software. Numeric variables were expressed as mean plus or minus standard deviation (SD), categorical variables were expressed as a percentage. The differences between two numerical variables were analyzed by the ANOVA test, the differences between categorical variables, using the CHI-SQUARED test. It was considered a statistically significant a P value <0.05. ROC curves were drawn to determine which value of TFC had the best significance and specificity to differentiate normal individuals from patients with TS.
Results
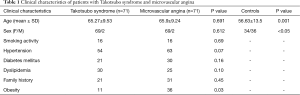
We enrolled 71 consecutive patients with TS, 69 women (97.18%) and 2 men (2.82%), with a mean age of 65.27±9.53 years, admitted to the Cardiology Unit of University Hospital Paolo Giaccone (n=27), Cervello Hospital (n=19) and of Ingrassia Hospital of Palermo, Italy (n=25) from 2007 to 2014. Moreover we enrolled 70 controls, 34 women (48.57%), and 36 men (51.43%), with a mean age of 56.63±13.5 years, without cardiovascular risk factors and coronary artery disease and 71 patients with MA, 69 women (97.18%) and 2 men (2.82%), with a mean age of 65.9±9.2 admitted to the Cardiology Division of the University Hospital P. Giaccone. Clinical characteristics of the patients with TS are summarized in Table 1.
Full table
The average LVEF was 42%±14.75% at admission and 59.5%±0.13% at discharge. Wall motion analysis showed an apical ballooning in all the examined TS patients and there was no evidence of atypical variants. The mean troponin peak was 6.05±6.86 ng/mL. In all patients, the coronary angiography excluded the presence of hemodynamically significant stenosis, occlusion and / or plaque rupture.
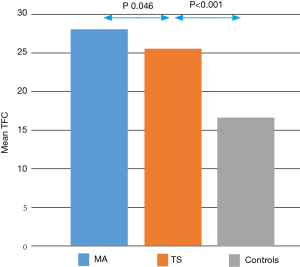
The assessment of the microcirculation, carried out through the TFC, showed significantly altered values in patients with TS compared with healthy controls. Comparing the average TFC for each vessel in patients with TS than in healthy controls, it was significantly higher in the former (LAD 25.16±6.91 vs. 17.30±3.76, P<0.001; CX 25.48±6.10 vs. 17.05±4.60, P<0.001; RCA 26.43±8.95 vs. 15.74±4.27, P<0.001. Table 2). The same result was confirmed by comparing the average TFC values found on all three vessels between patients with TS and controls (average TFC 25.70±5.34 vs. 16.70±3.26, P<0.001, Figure 1, Table 2).

Full table
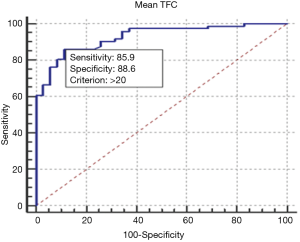
The ROC curve analysis showed that a TFC >20 frames is able to discriminate patients with TS from healthy controls with a specificity of 88.57% and sensitivity of 85.92% (AUC 0.927, P<0.0001, Figure 2).
The alteration of the microcirculation was diffuse in TS, in fact, there were no statistically significant differences in the comparison of TFC values between the three main vessels and it didn’t coincide with the territory perfused by a single epicardial coronary artery, consistently with the extent of wall motion abnormalities evaluated by echocardiogram.
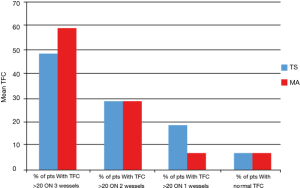
Specifically, a TFC value above >20 frames in all three vessels was found in 47% of patients (n=33), while in 28% of patients (n=20) impaired values were found simultaneously on two vessels and in 18% of cases (n=13) in a single vessel. In 5 patients (7%), we did not find values of TFC >20 frames in any vessel.
Considering as widespread a microcirculatory alteration in at least 2 vessels, we noticed that this was present in the 80.3% of patients with TS.
After confirming, by comparison with healthy controls, that a microvascular dysfunction is certainly demonstrable in patients with a diagnosis of TS, we compared the characteristics of microvascular impairment in TS and in MA. The mean TFC was slightly higher in patients with MA than in TS (TFC in MA 28.25±9.3 vs. 25.7±5.34 in TS, P<0.046, Figure 1).
The alteration was diffuse, across the coronary bed, also in MA patients, as shown in Table 2.
A TFC value above 20 frames in all three vessels was found in 57.74% of patients (n=41), while in 28.17% of patients (n=20) impaired values were found simultaneously on two vessels and in 7.04% of cases (n=5) in a single vessel. In 5 patients (7.04%), we did not find values of TFC >20 frames in any vessel. Figure 3 shows the distribution of TFC >20 frames across vessels in MA and TS.
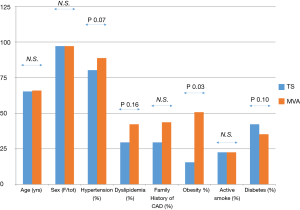
The prevalence of cardiovascular risk factors in TS and MA was not significantly different except for obesity, that is known to be strictly associated with MA (Figure 4). At multivariate analysis, the variables that were significantly associated with a TFC impairment were TS (OR 6.21, IC 95%: 1.2–32.1, P=0.029) and arterial hypertension (OR 9.38, IC 95%: 2.33–37.77, P=0.0016).
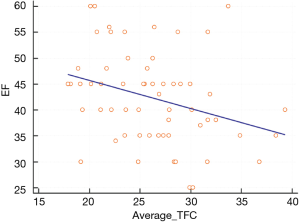
We found a linear relation between EF and average TCF in TS (Figure 5).
Discussion
The pathophysiology of TS is incompletely understood, and microvascular dysfunction is suggested as a pathogenetic hypothesis. Several studies have demonstrated the presence of impaired coronary microcirculation in patients with TS using different diagnostic tools such as angiography, nuclear cardiology, and echocardiography, however, the exact mechanism by which coronary microvascular dysfunction occurs remain unclear and particularly it is unclear if it is a causative factor or a bystander.
Some studies recorded alterations of the coronary microcirculation, at TFC analysis observing the coronary angiograms during the acute phases of the TS (13-18). Kume et al. detected the dysfunction of the coronary microcirculation with Doppler flow-wire technique in patients with TS (19). Other authors demonstrated, in patients with TS, abnormalities of the microcirculation through myocardial perfusion scintigraphy with technetium 99 m tetrofosmin (20-22). A disorder of glucose and fatty acid metabolism, even more, severe of the perfusion defect, has also been demonstrated (23-24). Abnormalities of coronary microcirculation have been also detected using contrast echocardiography (25-26). In accordance with angiographic studies, these authors demonstrated that they occur in the acute phase and typically recover at the resolution of the syndrome, even before wall motion abnormalities.
Most of the studies compared the characteristics of microcirculatory impairment in TS with acute coronary syndromes and healthy controls. They showed that the impairment of microvascular perfusion in TS is usually diffuse and not limited to the territory of a single epicardial coronary artery (13), microcirculation is more impaired in patients with TS than in controls and in patients with STEMI exhibiting myocardial reperfusion, while it is less impaired than in patients with STEMI exhibiting microvascular occlusion (27).
According to our knowledge, this is the first study that compared the characteristics of microcirculation in patients with TS and with MA. In a previous report, Takahashi et al. described the occurrence of TS in patients with pre-existing MA and they postulated that it could predispose to TS and it accentuates during its course (28). In contrast with this hypothesis, it must be pointed out that in most of the cases TS occurs in old women without any previous history of MA and that it has been demonstrated the microvascular impairment recovers after the resolution of the syndrome (25,26).
Accordingly, none of our patients had a history of MA preceding TS. The prevalence of cardiovascular risk factors, except for obesity, that was more frequent in MA, was not different is TS and MA, and for this reason, the microvascular dysfunction is probably proper of the TS and not secondary to the presence of cardiovascular risk factors.
The coronary microcirculation dysfunction in TS was found to be mild and wide spread, usually involving at least two vessels.
We detected cut-off values (>20 frames) to discriminate between patients with normal microcirculation and patients with TS. The microcirculatory abnormalities in TS were milder than those observed in patients with MA; we did not find a cut-off value able to discriminate between MA and TS.
The main limitation of our study is that we used to assess microcirculatory function indirect methods that are less accurate in defining microcirculatory impairment compared with other methods such as PET, MRI or contrast echocardiography, that unfortunately, it was not possible to perform. It is advisable to use these more precise techniques in future studies to better define microvascular involvement in TS compared to MA.
Conclusions
Our study confirms that in patients with TS there is a microvascular impairment, detectable by TFC. This dysfunction is mild and a cut off value of TFC >20 frames has high sensitivity and specificity in discriminating patients with TS from controls. We demonstrated that microvascular abnormalities are usually widespread, as well as in patients with MA, and not involving the territory of a single epicardial coronary artery, in accordance with the extension of wall motion abnormalities. Compared with MA, the amount of microvascular dysfunction is slightly lower.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethic committee approval was not requested because the study was observational and retrospective, it did not subjected patients to additional risks and it did not modify the normal clinical practice. Every patients signed informed consent for the use of their clinical data.
References
- Novo S, Akashi Y, Arbustini E, et al. Takotsubo cardiomyopathy: a consensus document. G Ital Cardiol (Rome) 2008;9:785-97. [PubMed]
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807-16. [Crossref] [PubMed]
- Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;118:2754-62. [Crossref] [PubMed]
- Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11-8. [Crossref] [PubMed]
- Kida K, Akashi YJ, Fazio G, et al. Takotsubo cardiomyopathy. Curr Pharm Des 2010;16:2910-7. [Crossref] [PubMed]
- Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the heart failure association of the European society of cardiology. Eur J Heart Fail 2016;18:8-27. [Crossref] [PubMed]
- Sato H, Tateishi H, Uchida T, et al. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, eds. Clinical aspects of myocardial injury: from ischemia to heart failure. Tokyo: Kagakuhyouronsha, 1990:56-64.
- Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472-9. [Crossref] [PubMed]
- Assennato P, Alfano R, Novo G, et al. Two cases of tako-tsubo cardiomyopathy in Caucasians. Ital Heart J 2005;6:614-7. [PubMed]
- Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart 2003;89:1027-31. [Crossref] [PubMed]
- Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408-17. [Crossref] [PubMed]
- Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879-88. [Crossref] [PubMed]
- Fazio G, Sarullo FM, Novo G, et al. Tako-Tsubo cardiomyopathy and microcirculation. J Clin Monit Comput 2010;24:101-5. [Crossref] [PubMed]
- Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J 2006;152:469.e9-13. [Crossref] [PubMed]
- Khalid N, Iqbal I, Coram R, et al. Thrombolysis in myocardial infarction frame count in Takotsubo cardiomyopathy. Int J Cardiol 2015;191:107-8. [Crossref] [PubMed]
- Khalid N, Iqbal I, Ikram S. Thrombolysis in myocardial infarction frame count in Takotsubo cardiomyopathy. J Am Coll Cardiol 2013;61:E50. [Crossref]
- Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004;94:343. [Crossref] [PubMed]
- Sharkey SW, Lesser JR, Menon M, et al. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (tako-tsubo) cardiomyopathy and comparison to those in patients with ST-elevation anterior wall myocardial infarction. Am J Cardiol 2008;101:1723-8. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary microcirculation in patients with Takotsubo-like left ventricular dysfunction. Circ J 2005;69:934-9. [Crossref] [PubMed]
- Abe Y, Kondo M, Matsuoka R, et al. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 2003;41:737-42. [Crossref] [PubMed]
- Nishikawa S, Ito K, Adachi Y, et al. Ampulla (‘Takotsubo’) cardiomyopathy of both ventricles: evaluation of microcirculation disturbance using 99mTc-tetrofosmin myocardial single photon emission computed tomography and doppler guide wire. Circ J 2004;68:1076-80. [Crossref] [PubMed]
- Ito K, Sugihara H, Katoh S, et al. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT--comparison with acute coronary syndrome. Ann Nucl Med 2003;17:115-22. [Crossref] [PubMed]
- Yoshida T, Hibino T, Kako N, et al. A pathophysiologic study of Takotsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J 2007;28:2598-604. [Crossref] [PubMed]
- Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003;41:743-8. [Crossref] [PubMed]
- Galiuto L, De Caterina A, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J 2010;31:1319-27. [Crossref] [PubMed]
- Jain M, Upadaya S, Zarich SW. Serial evaluation of microcirculatory dysfunction in patients with Takotsubo cardiomyopathy by myocardial contrast echocardiography. Clin Cardiol 2013;36:531-4. [Crossref] [PubMed]
- De Caterina AR, Leone AM, Galiuto L, et al. Angiographic assessment of myocardial perfusion in Tako-Tsubo syndrome. Int J Cardiol 2013;168:4717-22. [Crossref] [PubMed]
- Takahashi H, Tani S, Kikushima K, et al. Takotsubo cardiomyopathy in two patients with microvascular angina. J Cardiol Cases 2015;12:26-9. [Crossref]