The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer
Introduction
Lung cancer is still the leading cause of cancer death in China (1), for those patients with early stage non-small cell lung cancer (NSCLC), complete resection remains the standard treatment with unfavorable prognosis (2,3). However, the incidence rate of postoperative pulmonary complications (PCs) ranging from 15% to 37% for patients who undergo lobectomy (4-6), in which prolonged air leak and pneumonia are the most common (7-9). PCs prolong hospitalization, ICU care, increased medical cost (9,10), but limited researches focus on the potential impact of PCs on long-term survival for lung cancer patients (11).
To date, though video-assisted thoracic surgery (VATS) lobectomy has been proved to be more safe and reliable than thoracotomy because of its obvious minimally invasive advantages, quicker postoperative recovery, less postoperative complications, especially for the elderly patients with low pulmonary function or comorbidities (12-16), PCs are still inevitable, therefore, accurate prediction of PCs is of great clinical significance, which can improve the quality of life and long-term survival of lung cancer patients. However, most of the studies concerned about the presence of PCs, without the severity of the complications. It is vital to evaluate and determine the risk factors of major pulmonary complication (MPC) objectively.
In this study, we sought to determine the long-term effect of MPCs, develop and validate a nomogram predictive of them. Our study aims were: (I) to assess the long-term influence of MPCs in NSCLC patients who underwent VATS lobectomy; (II) to identify risk factors for MPCs.
Methods
Patients and study design
A retrospective study was conducted among the primary NSCLC patients who underwent VATS lobectomy at the Peking University People’s Hospital (PUPH) between January 2007 and December 2015, a cohort of 828 patients were identified who satisfied the following inclusion criteria: (I) preoperative clinical stage must be I–II; (II) age >18 years old; (III) physical status ECOG score of 0–1. Exclusion criteria were as follows: (I) pathologically confirmed as adenocarcinoma in situ (AIS); (II) patients who accepted neoadjuvant chemotherapy or radiation; (III) patients who had received thoracic surgery before, except for previous diagnostic thoracoscopic surgery; (IV) history with malignant tumors in the last 5 years. We explore the impact of MPCs on the long-term prognosis and identify the independent risk factors for MPCs. The ethical review and informed consent of this study were approved by institutional ethics board of Peking University People’s Hospital (No. 2014PHB033-01).
Data collective
We collected clinical variables of patients including: demographics [age, sex, comorbidities, smoking status, pulmonary function, American Society of Anesthetist (ASA) score]; surgical data (estimated blood loss, surgery time, numbers of dissected lymph nodes and dissected lymph nodes stations); pathological data (histology type, pathologically positive number of lymph nodes and number of stations, TNM stage); postoperative data [length of stay (LOS), drainage time, etc.].
Chest CT scan and abdominal ultrasound/CT are performed on follow-up visits every 6 months, after operation for 5 years. MRI and bone scan are performed every 1 year for 5 years or any time with symptoms. The overall survival (OS) was estimated from the date of surgical resection until death of any cause or the date of last follow-up. Disease-free survival (DFS) was defined as the time from the day of surgery until the first event (relapse, metastasis or death from any cause) or last follow-up.
Complications
Perioperative mortality was defined as death at the time of hospitalization or within 30 days after surgery. Postoperative complications were defined and graded according to the TM&M classification (17) and the common terminology criteria for adverse events (CTCAE 4.0), which grades complications on a severity scale from grades I to V based on the effort required to treat the events. Grades I and II include events that deviate from the normal postoperative course but require either no intervention or pharmacologic therapy, respectively, which were defined as minor complication. Grade III and IV complications were defined as major complication, a grade III event required medical intervention, without general anesthesia (IIIa), and with general anesthesia (IIIb). Grade IV events were life-threatening and require intensive care unit management owing to single organ dysfunction (IVa) or multi organ dysfunction (IVb). Grade V events resulted in death of the patient.
VATS technique
Under single-lung anesthesia, a patient was placed in the lateral decubitus position with an air pillow underneath, and the upper extremities were extended forward. A 30-degree thoracoscope was placed in the 7th intercostal space (ICS) at the midaxillary line; the working port (often 4–5 cm) was placed in the 4th ICS at the anterior axillary line; the assistant’s port was put in the 7th or 8th ICS between the posterior axillary and subscapular lines. The process and skills of VATS lobectomy followed the operational guidelines of Peking University People’s Hospital described in 2010 (18). The main points include: (I) a specially made curved aspirator and electrocautery were used concurrently through working port and double-crossed in the same direction, known as Wang’s technique; (II) deal with the bronchial artery firstly in the hilum through coagulation or ligation; (III) pulmonary artery and pulmonary vein are freed in the subadventitial plane and transected by endo stapler after removing the surrounding lymph nodes to “vascular skeletalization” status; (IV) for smaller vessels, we can also use Hem-o-Lok automatic ligation clip, ligasure bipolar electric knife and suture. All lung cancer patients would undergo systematic mediastinal lymph node dissection.
Statistical analysis
Continuous variables were compared using Student’s t-test or Mann-Whitney non-parametric test for variables with an abnormal distribution, frequency distributions were compared using the chi-square test or Fischer’s exact test. Kaplan-Meier method and the log-rank test were used to assess the impact of PCs on survival. Univariable and multivariable survival analysis was performed using the Cox proportional hazards model, with results presented as hazard ratio (HR) with 95% confidence interval (CI). A P value of <0.05 was considered to represent a statistically significant difference. Statistical analysis was performed using SPSS v.22.0 (IBM, Chicago, IL, USA).
Results
Clinicopathologic characteristics of patients
There were 828 lung cancer patients who underwent VATS lobectomy during the period, 417 (50.4%) of who were male. The age of the group was 64.5±10.3 years. Mean body mass index (BMI) was 24.0±3.1 kg/m2. No intraoperative deaths occurred. The median operation time was 170 min (range, 140–206 min), with the estimated blood loss of 50 mL (range, 50–100 mL). Most common histology type was adenocarcinoma (82.9%), followed by squamous cell carcinoma (13.2%) and other type (4%). There were 9 perioperative deaths (1.1%). Patient characteristics are shown in Table 1.

Full table
Postoperative PCs
A total of 139 patients (16.8%) had clinical evidence of a PC, in which 75 patients (9%) had MPCs. The most common PCs included prolonged air leak (7.5%), pneumonia (5%), pleural effusion (3.1%) and pulmonary atelectasis (1.4%). The details of PCs are listed in Table 2. As is shown in Table 3, patients who suffer from postoperative PCs or MPCs had longer median hospital LOS and drainage time. In the PCs group, there was also higher perioperative mortality rate.
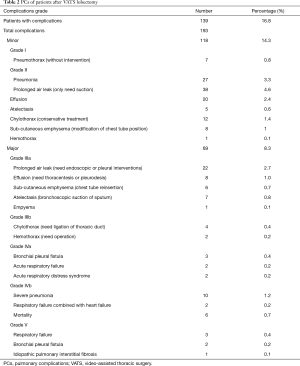
Full table

Full table
Impact of MPCs on long-term outcomes
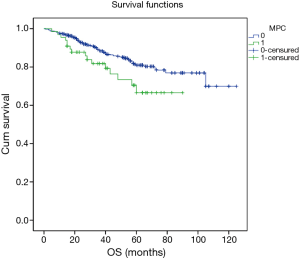
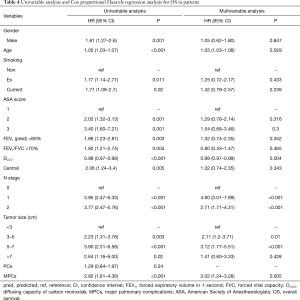
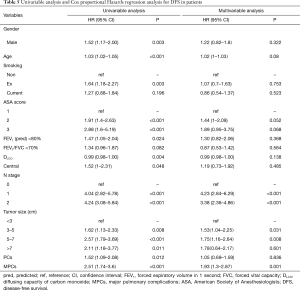
Excluding 9 patients who died of a postoperative complication, of the remaining 819 patients, there were 128 (15.5%) deaths with a median follow-up of 41 months (rang, 22–59 months). Those who developed a postoperative pulmonary complication (PPC) presented a tendency of decrease in 3-year DFS and 5-year DFS (74.7% vs.78.4%, 64.3% vs. 69.4%; P=0.088) (Figure 1), as well as a tendency of decrease in 3-year OS and 5-year OS (86.8% vs. 88.2%, 78.3% vs. 80%; P=0.893) (Figure 2), there was no significant difference in long-term survival between the two groups. However, those who develop a MPC had a reduced 3-year DFS and 5-year DFS (68.2% vs. 78.7%, 44.7% vs. 70.3%; P=0.001) (Figure 3), as well as the reduced 3-year OS and 5-year OS (81.8% vs. 88.6%, 66.6% vs. 80.9%; P=0.023) (Figure 4). Using multivariate analysis, the significant risk factors associated with late deaths in lung cancer patients were MPCs (HR: 2.02, 95% CI: 1.24–3.28; P=0.005), age (HR: 1.05, 95% CI: 1.03–1.08; P=0.029), diffusing capacity of carbon monoxide (DLCO) (HR: 0.98, 95% CI: 0.97–0.99; P=0.004), and N stage and tumor diameter (Table 4). Significant independent risk factors for recurrence in patients with NSCLC were MPCs (HR: 1.93, 95% CI: 1.3–2.87; P=0.001), N stage and tumor diameter (Table 5).
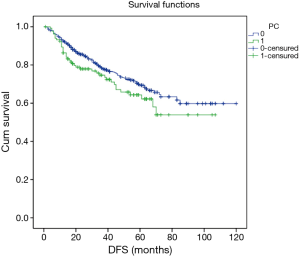
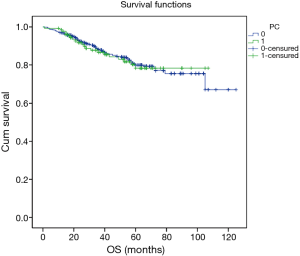
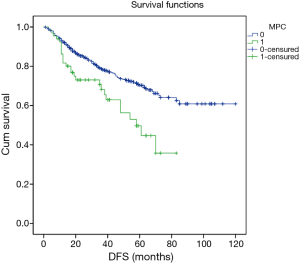
Full table
Full table
Risk factors for MPCs
Multivariate analysis demonstrated that age (P=0.007, HR: 1.05, 95% CI: 1.01–1.08), male (P=0.001, HR: 3.33, 95% CI: 1.87–5.94), ASA grade were independent risk factors for MPCs in primary cohort (Table 6).

Full table
Discussion
Although complete surgical resection is still the standard treatment for patients with lung cancer, the 5-year overall survival rate after lobectomy is currently ranging from 57% to 82% (4,5). Surgical and perioperative management improvements reduce postoperative mortality, But the incidence of PCs is still very high, ranging from 17% to 37% (11,19), which lead to prolonged hospitalization, ICU stay, increased rate of rehospitalizations, as well as significant increased perioperative mortality. Previous studies on the prognosis of lung cancer are often focused on tumor size, lymph node metastasis, distant metastasis, chemotherapy sensitivity and other clinical pathology features (20). In recent years, more and more research began to focus on the patient’s own factors, such as: the patient’s physical condition, immune status, depression (21). However, the impact of perioperative complications on the outcome of the lung cancer patients has been seldom studied (11), this study is the first to determine the association between MPCs and long-term outcomes among patients undergoing VATS lobectomy for NSCLC. Excluding perioperative deaths, MPC is an important independent risk factor for recurrence and long-term death of lung cancer, which provides an understanding new insight of the important clinical and pathological features associated with cancer.
The frequency of PCs (16.8%) in our study was consistent with previous studies (17–37%). They reported a significant difference in the incidence of PCs after lung surgery due to the absence of a standard definition of PCs. In this study, TMM classification and CTCAE4.0 were used to define and grade all PCs, which made the results more reliable and practical.
Our study showed that OS and RFs are reduced in patients who developed a MPC after VATS lobectomy for NSCLC. There are few studies on the effect of MPC on the long-term prognosis of lung cancer. A retrospective study conducted by Rueth in 2011 which include patients who have underwent lobectomy for stage I NSCLC shows that, patients with postoperative complications were associated with poorer cancer-specific survival (70.9% vs. 78.9%; P<0.001) and reduced 5-year OS (52.7% vs. 65.9%, P<0.001), postoperative complication is an independent risk factors for mortality (HR: 1.46, 95% CI: 1.24–1.73) (22). In a prospective observational study involved 670 lung resections in 2016, Sebastian found that COPD, smoking were independent risk factors for PCs, those who developed a PPC resulted in a significantly reduced overall survival (40 vs. 46; P=0.006) and higher rate of non-cancer-related deaths (11% vs. 5%; P=0.020). PPC is a significant independent risk factor for late deaths in NSCLC patients (HR: 2.0, 95% CI: 1.9–3.2; P=0.006) (11). In our study, we not only evaluated the presence of PC, but also the severity of them, we find that developing a MPC after VATS lobectomy is associated with poorer long-term prognosis. These data suggest that, in addition to general systemic inflammatory response itself, complications, particularly major complications, may further exacerbate inflammatory and immunosuppressive states, resulting in the negative impact on the long-term outcomes.
Male and elderly patients are more likely to developing MPCs after lung resection. Fernandez et al. [2016] indicated that being male and elderly patients have consistently been identified as negative predictors of major postoperative complications after lung cancer surgery (23). Perioperative mortality in male patients was also significantly increased. Tong et al. also found a significant increase in hospitalization stay after lung resection in male patients, possibly because that men were less tolerant of pain and were often associated with more comorbidities (24). Elderly patients are often accompanied by more comorbidities, hence the risk of postoperative complications increases. However, Dominguez-Ventura et al. indicated that elderly lung cancer patients could still benefit from radical lobectomy, the mortality was only 0–8.8%, but with higher rate of postoperative complications, but postoperative complications increased significantly with age, our study also shows this trend (25). Rueth et al. showed that complications in patients over 75 years old after lobectomy was 1.3 times more compared with patients less than 70 years old, the further stratification analysis showed that age was the independent risk factor for cardiac complications, but not a risk factor for PCs and other complications (22).
Nicholas firstly proposed that ASA score has a strong ability to predict surgical complications and perioperative mortality (21), and then Agostini showed that the risk of PCs increased by 3.9 times in patients with ASA score ≥3 after lung resection (4), our study also shows that ASA score was associated with MPCs in NSCLC patients after VATS lobectomy.
There are several limitations to this study. First, because of its retrospective nature, there is inevitable bias in the data collection and assessment of postoperative complications. In addition, there is a relatively short follow-up time in the analysis of impact on long-term outcomes.
Conclusions
In summary, we found that MPCs after VATS lobectomy were associated with a poorer long-time outcome in lung cancer patients. Age, male, ASA score were the independent risk factors of MPCs.
Acknowledgements
Funding: The authors would like to thank Beijing Municipal Science and Technology Plan for the support of this study (D141100000214004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Peking University People’s Hospital (No. 2014PHB033-01) and written informed consent was obtained from all patients.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014;121:219-31. [Crossref] [PubMed]
- Canet J, Gallart L. Predicting postoperative pulmonary complications in the general population. Curr Opin Anaesthesiol 2013;26:107-15. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Shander A, Fleisher LA, Barie PS, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 2011;39:2163-72. [Crossref] [PubMed]
- Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006;144:581-95. [Crossref] [PubMed]
- Canet J, Mazo V. Postoperative pulmonary complications. Minerva Anestesiol 2010;76:138-43. [PubMed]
- Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171-6. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, aff ordable, and timely cancer surgery. Lancet Oncol 2015;16:1193-224. [Crossref] [PubMed]
- Amar D, Munoz D, Shi W, et al. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer. Anesth Analg 2010;110:1343-8. [Crossref] [PubMed]
- Beilin B, Bessler H, Mayburd E, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology 2003;98:151-5. [Crossref] [PubMed]
- Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 2015;18:184-90. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. The long-term impact of surgical complications after resection of stage I nonsmall cell lung cancer: a population-based survival analysis. Ann Surg 2011;254:368-74. [Crossref] [PubMed]
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- Tong BC, Kosinski AS, Burfeind WJ, et al. Sex differences in early outcomes after lung cancer resection: analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg 2014;148:13-8. [Crossref] [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung Cancer in Octogenarians: Factors Affecting Morbidity and Mortality After Pulmonary Resection. Ann Thorac Surg 2006;82:1175-9. [Crossref] [PubMed]


