Primary breast leiomyosarcoma and synchronous homolateral lung cancer: a case report
Introduction
Primary mesenchymal tumors of the breast are uncommon and they are best stratified into phyllodes (more common, often benign) and sarcomas (rare, always malignant) varieties. Breast sarcomas account for less than 1% of breast tumors and comprise malignant phyllodes tumors, sarcomas arising in the post-irradiated breast and soft tissue sarcomas of different histotypes; of the latter, cysto-sarcoma phyllodes is the most common one, while leiomyosarcomas are one of the less common subgroup, representing the 2.5–6% (1-6).
Breast leiomyosarcomas usually present as enlarging palpable masses, usually painless, firm, and lobulated (1-5). There are no pathognomonic mammographic or ultrasonographic features. Therefore, fibroadenoma, pseudo-angiomatous stromal hyperplasia (PASH) and phyllodes are the main differential diagnoses at the breast conventional imaging (7,8). Rare malignant tumors, including angiosarcoma, medullary or mucinous carcinoma, poorly differentiated carcinoma or metaplastic breast carcinoma may be considered as well.
Even though imaging plays a fundamental role within the first diagnostic approach to breast lesions, final diagnosis is confirmed only by histological examination and immunohistochemical analysis.
Hematogenous spread is the most common path of dissemination and occurs typically in lungs, pleura and/or bones (4,5). Surgical resection remains the gold standard of treatment, whereas radiation therapy (9) and chemotherapy have a limited role (4,5).
Perioperative imaging for distant metastases is performed in newly diagnosed breast leiomyosarcoma patients, uncovering incidental findings of uncertain significance. In our case, the staging CT scan depicted a lung lesion for which differential diagnosis work-out was essential in the definition of an adequate therapeutic approach.
Case presentation
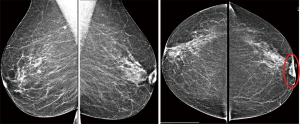
A 62-year-old woman with no prior health issues presented to our Breast Unit with a mass in the retroareolar region of the left breast. The patient had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of with no prior oncological history and no previous breast or lung disease. Physical examination revealed a soft and mobile small lump without associated skin changes in the retroareolar left breast but there was a minimal nipple shrinkage. The lump appeared 3 years earlier during breast screening and misinterpreted as a benign finding. In fact, in this region of the left breast, the ultrasound performed three years earlier showed a 5-mm sized lesion assessed as Breast Imaging-Reporting and Data System (BI-RADS) category 2. Even mammography was unremarkable due to its superficial position under the retroareolar skin and due to its small dimensions. It was recommended regular radiological follow-up and clinical observation that the patient had not being performing during the following three years. However, after this period, the lesion increased in size and became well palpable and, therefore, the patient underwent conventional imaging, firstly. Digital mammograms showed a single, oval, high-density lesion with well-defined margins without microcalcification associated that corresponded to the palpable finding; it was also associated to nipple shrinkage (Figure 1).
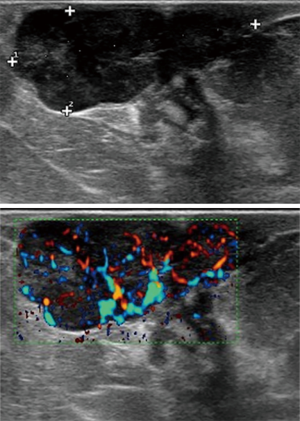
The ultrasound examination confirmed a solid nodule (dimension of 27 mm × 10 mm), highly vascularized at the color Doppler, with oval shape, well-defined margins and slightly heterogeneous echostructure (Figure 2). The lesion extended into the overlying subcutaneous fatty tissue without expressing any acoustic shadow. The findings were suggestive for a neoplasm without a clear evidence of a benign or malignant nature. No suspicious axillary lymph nodes were found at the ultrasound examination.
The ultrasound-guided aspiration with fine needle didn’t demonstrate any malignant cytological features. Even the needle-biopsy was unsatisfactory for diagnosis. For these reasons and for the very suspicious clinical characteristics of the breast lesion (nipple alteration and increase in dimensions), the patient underwent a diagnostic partial breast resection that demonstrated a malignant lesion compatible with neoplasm originated by smooth muscle tissue such as leiomiosarcoma. Pathological evaluation revealed a malignant mesenchymal neoplasm composed of pleomorphic spindle cells, with high mitotic rate [up to 5 mitosis/10 high power fields (HPF)] and proliferation index (Ki67: 50%), expressing smooth muscle markers, consistent with leiomiosarcoma.
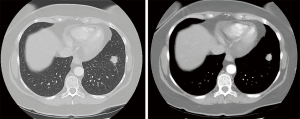
According to the histological diagnosis, a staging CT scan was performed. It confirmed the presence of a soft tissue mass involving the left breast (about 3 cm in the major diameter) with no chest wall invasion and a suspicious lung nodule in the lower left lobe (about 2 cm). The CT images showed a mass with irregular borders with inhomogeneous contrast enhancement (Figure 3); there was not lymph node with malignancy radiological features. No abdominal lymphadenopathy, peritoneal or liver metastases were identified.
In order to discriminate between a primary lung lesion and a single lung metastasis from breast leiomyosarcoma, a preoperative positron emission tomography (PET/CT) scan was performed and showed a large 18F-fludeoxyglucose (18F-FDG) avid mass in the lower left lung lobe corresponding to the CT findings; the left breast lesion was also demonstrated.
Both PET/CT imaging and thorax-abdominal CT scan did not reveal other pathological findings. Therefore, the patient underwent a bronchofiberscopy with bronchial aspirate and lavage, which were both negative for malignancy. The patient was referred to our Institution for definitive treatment.
A CT-guided fine needle biopsy of the lung nodule was performed and revealed a primary lung adenocarcinoma at light microscopic examination. Patient was in good general health conditions, laboratory tests were unremarkable, and the pre-surgical work-out showed no contraindications to open major thoracic surgery associated at the homolateral breast conservative surgery. The pre-surgical tests such as transthoracic echocardiogram, electrocardiogram and spirometry showed no real risk factors.
The patient underwent curative left lower lobectomy and lymphadenectomy through anterolateral thoracotomy and, simultaneously, left central quadrantectomy removing the previous incision and nipple/areola complex (NAC). Surgical margins were determined by macroscopic and histologic examination of frozen sections of the breast specimens in the operating room. An adequate safety margin of 1 cm was obtained. Breast reconstruction was done using round block technique with a good cosmetic outcome. Since lymphatic spread and nodal metastasis are not typical features of leiomyosarcomas, sentinel lymph node biopsy or axillary lymph node dissection were not performed.
Postoperative course was uneventful and the patient was discharged on day 4 after operation. On gross examination of the lung specimen, a 2 centimeters subpleural nodule was described. At microscopic level, no necrosis nor vascular invasion were found. Lymph nodes were all negative.
Histological diagnosis was a moderately differentiated (G2) lepidic adenocarcinoma (pT2aN0Mx, according to the TNM 7th edition) of the lung, focally invading the visceral pleura.
Histological diagnosis of the breast lesion was high grade leiomiosarcoma [G3 according to Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC)] with the following immunophenotype: SM-actin+, desmin+, caldesmon+, vimentin+, estrogen receptor+, S100-, FLi1-, ERG-, CKpool-, progesteron receptor-, p63-, CK7-, CAM5.2-, CK34, betaE12-, CD10-, ERG-, CD31-. The proliferation index MIB1 was 45%. The margins of the surgical specimen were wide and free even by microscopic examination.
Considering the histological subtype of soft tissue sarcoma (leiomyosarcoma), its high FNCLCC grading and its limited dimensions (2-cm nodule), it was performed whole breast adjuvant radiotherapy in order to reduce the risk of local relapse. No adjuvant chemotherapy was delivered.
Discussion
We have reported the case of a 62-year-old woman with a very rare diagnosis of a primary breast leiomyosarcoma presenting with a synchronous ipsilateral lung lesion.
The exact origin of breast leiomyosarcomas is debated. They belong to the subgroup of ‘spindle cell’ breast tumors which generally arise either from the smooth muscle cells lining blood vessels or from stromal mesenchymal cells, thus reflecting the controversy surrounding the aetiology of these tumors. Since most of them are located near the areola (such as in our case), they are likely to origin from blood vessels and musculature of this anatomical region (9,10).
In breast sarcomas, skin and nipple areola are rarely involved but the latter was involved in our case (1-5,10).
Some evidence suggests that these tumors are slowly growing because most patients, as in our case, reported a palpable mass several years before seeking treatment (3 years in our case) (1-5,10). Typically, there are no abnormal serologic values or tumor markers in these patients, such in our case (1-5,10).
In our case, the lung lesion was an incidental finding during pre-surgical staging exams, mimicking a secondary localization of the mesenchymal breast neoplasm.
Indeed, hematogenous spread is the most common route of initial metastasis for sarcomas, which occurs mostly in the lungs, pleura, and bones. Furthermore, for cases with single lung lesions, pulmonary metastasectomy is a validated therapeutic option in selected patients with soft tissue sarcomas.
Here, we have prevalently discussed the leiomyosarcoma diagnosis and the differential diagnosis for the lung lesion at disease presentation, since this was the relevant clinical aspect, since leiomyosarcoma was the tumor which could change the patient’s prognosis. Indeed, a stage IB lung adenocarcinoma has a 5-year overall survival (OS) of 45–54% after surgery, while metastatic sarcomas patients have a poorer prognosis with a 3-year OS of about 20–30%. Our patient had a lung lesion with typical radiological and morphological features of a primary lung cancer. The CT-imaging characteristics (new and single lesion, with irregular shape and ill-defined margins) and the positive uptake of 18F-FDG of the mass made it highly suspicious for a primary lung cancer rather than a single metastasis from leiomyosarcoma (typically showing as multiple lesions, with regular and oval shape and well-defined margins) as it could had been suggested by the sarcomatous histotype of the breast lesion (previous excisional biopsy had demonstrated its sarcomatous origin).
Primary breast sarcoma is easily confused with fibroadenoma on ultrasound examinations and on cytological analysis (7,8,11,12), as seen in our patient. In particular, the first ultrasound examination, performed three years earlier when the physical examination revealed a soft and mobile small lump in the retroareolar left breast, had demonstrated a benign finding probably attributable to a fibroadenoma or a dense cyst. Moreover, also in our case the fine needle aspiration cytology procedure was unremarkable.
However, despite the heterogeneity of primary breast sarcomas, the consequences of being falsely reassured by ultrasonographic misdiagnose and negative cytology are potentially more serious. Mammographic findings in sarcoma are also non-specific (5,7,8).
Primary breast sarcoma may appear as a non-spiculated dense mass with indistinct borders to areas of asymmetry, such as in our case, or even without mammographic abnormality. However, in our case, mammographic features were suggestive for a benign lesion.
Considering the radiological and ultrasonographic features only (high-density lesion without associated microcalcification, oval shape, well-defined margins, slightly homogeneous echostructure, absence of the acoustic shadow, and expansive pattern of growth), the diagnosis of a benign lesion could be erroneously made. Therefore, we recommend to integrate clinical examination and tru-cut core biopsy in specific cases to avoid misdiagnosis on the basis of the morphological features given by the ultrasonography only.
Immunohistochemical staining is useful and usually positive for smooth muscle actin and vimentin. Epithelial membrane antigen, keratin, and s-100 protein are inconsistently present.
Most of the data on breast leiomyosarcoma is based on case reports or small case series, and the natural history and response to different therapeutic modalities in this disease have not been well established. To our knowledge, there is no standard treatment algorithm in such cases. Surgical resection remains the cornerstone of the treatment, while radiation therapy and chemotherapy in the neoadjuvant, adjuvant or metastatic setting are yet undefined (5).
We performed a breast conservative surgery as suggested in few cases reported in literature. These studies showed no differences in outcome in patients with breast conservative surgery with microscopically negative margins compared with mastectomy (5,13-20).
Lymphatic spread is uncommon as shown by the absence of axillary lymph node metastasis in our case and other published ones, and therefore axillary node dissection is not necessary to be performed.
A breast leiomyosarcoma could present with distant metastases which constitute a typical sign of malignancy. In our case, the patient had a single pulmonary suspicious mass and a synchronous breast lesion thought to be two primary lesions due to the radiological features. This could potentially lead to a difficult diagnosis as the pulmonary mass may mimic a sarcoma pulmonary metastasis. There are rare cases of breast leiomyosarcoma and synchronous ipsilateral lung cancer.
The incidence of local recurrence, distant metastasis, and cancer-related death are relatively low but high grade leiomyosarcomas have an intrinsic risk of local relapse. Therefore, adjuvant radiotherapy is usually delivered after wide conservative surgery with negative margins in order to maximize local control and reduce the risk of local recurrence (14-23). However, guidelines regarding radiation therapy in breast sarcomas were not derived from randomized trials but extrapolated from extremity soft tissue sarcomas. On the other hand, very high-risk lesions (high grade leiomyosarcomas, larger than 5 cm) also present a high propensity for distant metastases, thus requiring a complex patient-physician shared decision-making on individual basis about risks and benefits of an adjuvant anthracycline-based chemotherapy (16-23). In our case, considering the limited extent of the disease, we did not recommend a systemic treatment.
Furthermore, response rates to systemic therapies remain poor, thus reinforcing the need for an accurate pre-operative diagnostic strategy, a multidisciplinary evaluation of every single case and an appropriate surgical management.
Conclusions
When encountering such cases we suggest considering the possibility of a breast leiomyosarcoma, because even though it is rare, the prognosis and treatment changes significantly.
Imaging techniques are the first fundamental tools to obtain an initial diagnosis based on the morphological and functional features of the breast leiomyosarcoma but the final diagnosis still remains the interventional approach.
Most of the data on breast leiomyosarcomas are based on case reports or small case series, and the natural history and response to different therapeutic modalities in this disease have not been well established. To the best of our knowledge, there is no standard treatment algorithm in such cases. Surgical resection remains the cornerstone of treatment, while radiation therapy and chemotherapy in the neoadjuvant, adjuvant, and metastatic setting remains to be defined on a single-patient basis.
Acknowledgements
We thank Dr. Gerardo Cioffi, native speaker, for reviewing the English language.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and accompanying images. A copy of the written consent is available for review by the First Author of this report.
References
- Al-Benna S, Poggemann K, Steinau HU, et al. Diagnosis and management of primary breast sarcoma. Breast Cancer Res Treat 2010;122:619-26. [Crossref] [PubMed]
- Hussien M, Sivananthan S, Anderson N, et al. Primary leiomyosarcoma of the breast: diagnosis, management and outcome. A report of a new case and review of literature. The Breast 2001;10:530-4. [Crossref] [PubMed]
- Adem C, Reynolds C, Ingle JN, et al. Primary breast sarcoma: Clinicopathologic series from the Mayo clinic and review of the literature. Br J Cancer 2004;91:237-41. [Crossref] [PubMed]
- Spitaleri G, Toesca A, Botteri E. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol 2013;88:427-36. [Crossref] [PubMed]
- Toesca A, Spitaleri G, De Pas T. Sarcoma of the breast: outcome and reconstructive options. Clin Breast Cancer 2012;12:438-44. [Crossref] [PubMed]
- Meroni S, Moscovici O, Menna S, et al. Ultrasound challenge: secondary breast angiosarcoma mimicking lipoma. Breast J 2013;19:437-8. [Crossref] [PubMed]
- Elson BC, Ikeda DM, Andersson I, et al. Fibrosarcoma of the breast: mammographic findings in five cases. AJR Am J Roentgenol 1992;158:993-5. [Crossref] [PubMed]
- Yang WT, Hennessy BT, Dryden MJ, et al. Mammary angiosarcomas: imaging findings in 24 patients. Radiology 2007;242:725-34. [Crossref] [PubMed]
- Barrow BJ, Janjan NA, Gutman H. Role of radiotherapy in sarcoma of the breast: a retrospective review of the M.D Anderson experience. Radiother Oncol 1999;52:173-8. [Crossref] [PubMed]
- Shinto O, Yashiro M, Yamada N, et al. Primary leiomyosarcoma of the breast: report of a case. Surg Today 2002;32:716-9. [Crossref] [PubMed]
- Gupta RK, Kenwright D, Narsan S, et al. Fine needle aspiration cytodiagnosis of leiomyosarcoma of the breast: a case report. Acta Cytol 2000;44:1101-5. [Crossref] [PubMed]
- Jun Wei X, Hiotis K, Garcia R, et al. Leiomyosarcoma of the breast: a difficult diagnosis on fine-needle aspiration biopsy. Diagn Cytopathol 2003;29:172-8. [Crossref] [PubMed]
- Kamio T, Nishizawa M, Aoyama K, et al. Primary leiomyosarcoma of the breast treated by partial resection of the breast including nipple and areola: report of a case. Surg Today 2010;40:1063-7. [Crossref] [PubMed]
- Zelek L, Llombart-Cussac A, Terrier P. Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. J Clin Oncol 2003;21:2583-8. [Crossref] [PubMed]
- Tan BY, Acs G, Apple SK, et al. Phyllodes tumours of the breast: a consensus review. Histopathology 2016;68:5-21. [Crossref] [PubMed]
- Holm M, Aggerholm-Pedersen N, Mele M, et al. Primary breast sarcoma: a retrospective study over 35 years from a single institution. Acta Oncol 2016;55:584-90. [Crossref] [PubMed]
- Borhani-Khomani K, Talman ML, Kroman N, et al. Risk of local recurrence of benign and borderline phyllodes tumors: a danish population-based retrospective study. Ann Surg Oncol 2016;23:1543-8. [Crossref] [PubMed]
- Blanchard DK, Reynolds CA, Grant CS, et al. Primary non phylloides breast sarcomas. Am J Surg 2003;186:359-61. [Crossref] [PubMed]
- McGowan TS, Cummings BJ, O'Sullivan B, et al. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys 2000;46:383-90. [Crossref] [PubMed]
- Hernanz F, Alvarez A, Ortega E, et al. Malignant cystosarcoma phyllodes of the breast treated with oncoplastic conservative surgery. Breast J 2005;11:146. [Crossref] [PubMed]
- Fields RC, Aft RL, Gillanders WE, et al. Treatment and outcomes of patients with primary breast sarcoma. Am J Surg 2008;196:559-61. [Crossref] [PubMed]
- Nizri E, Merimsky O, Lahat G. Optimal management of sarcomas of the breast: an update. Expert Rev Anticancer Ther 2014;14:705-10. [Crossref] [PubMed]
- Confavreux C, Lurkin A, Mitton N, et al. Sarcomas and malignant phyllodes tumours of the breast: a retrospective study. Eur J Cancer 2006;42:2715-21. [Crossref] [PubMed]


