Is single-station N2 disease on PET-CT an indication for primary surgery in lung cancer patients?
Despite development of novel systemic treatment and radiation strategies, surgery remains the most successful method of radical treatment of non-small-cell lung cancer (NSCLC). However, in patients with locally advanced disease it is recommended to combine surgery with chemotherapy, sometimes with an addition of radiation, to improve the cure rate and distant survival. Authors of the recently available article (1) which is the subject of our polemic compare late survival of two groups of patients with minimal N2 disease who are considered candidates to combined treatment with the predominant role of surgery according to current recommendations. Namely, they compare patients with occult mediastinal lymph node metastases (false negative N2 disease in preoperative positron emission tomography) with those with single-station clinical N2 (cN2) disease which was not confirmed microscopically before surgery. Due to the latter, in both groups of patients no preoperative chemotherapy was administered. This was not a usual situation because in most patients with suspicion of N2 disease, metastases to mediastinal lymph nodes can be confirmed preoperatively and only an “obscured” location of N2 lymph nodes in patients accrued to this study allowed authors to formulate the hypothesis and create the study protocol. According to current recommendations, patients with preoperatively confirmed N2 disease should receive neoadjuvant chemotherapy while in patients with metastases diagnosed only on the basis of postoperative specimen (so called occult metastases) adjuvant chemotherapy is recommended.
Authors conclude that mean overall survival and mean disease-free survival are similar in both groups. They even extrapolate these results from patients with “obscured” single-station N2 into all patients with single-station N2 disease. This extrapolation seems justified because prognosis of patients with N2 disease is similar regardless of the exact location of lymph nodes. When this conclusion is extrapolated into all patients with single station N2 disease, it rationalizes primary surgical treatment in a significant number of patients who nowadays are treated with neoadjuvant chemotherapy prior to surgery. Although only few direct comparisons of neoadjuvant and adjuvant chemotherapy are available (2,3), these two approaches seem to be comparable in terms of survival (4,5). Moreover, latest development of immunotherapy, molecularly targeted agents, biomarkers and radiation therapy speaks in favor of adjuvant treatment over neoadjuvant cytotoxic chemotherapy.
Numerous factors have been analyzed as predictors of occult metastases to mediastinal lymph nodes (not discovered by preoperative PET scan). Some of them have been confirmed as predictors of false-negative results of PET scan [Table 1 (6-15)]. Among these factors adenocarcinoma histology, right upper or middle lobe location of the primary tumor, high maximum standardized uptake value (SUVmax) of the primary tumor, large tumor diameter, vascular invasion, central location of tumor, positive 18F-FDG uptake in intrapulmonary and/or hilar lymph nodes and consolidation/tumor dimension ratio are best documented. These analyses mostly show the tendency of lung cancer to metastasize to ipsilateral mediastinal lymph nodes regarding tumor location (e.g., vascular invasion) and histology (biology) of the tumor. Application of two models of an Artificial Neural Network to predict metastases into mediastinal lymph nodes showed 89% and 92% accuracy with two factors (SUVmax >2.8 and lymph node length >15 mm) being most sensitive (16) but their value should be assessed in a larger and prospective analysis.
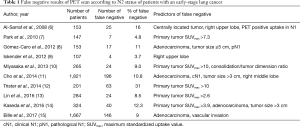
Full table
The analyzed paper aims to provide new data on the role of primary surgery in lung cancer patients with metastases to the ipsilateral mediastinum. This topic is a matter of ongoing debate, full of limitations and concerning a heterogeneous group of patients. Since prospective randomized trials (17,18) did not show any advantage of surgery combined with preoperative chemotherapy over chemoradiotherapy, before recommending primary surgery in any subgroup of patients with N2 disease a comparison of late results of such treatment with chemo-radiotherapy should be performed, at least on the basis of meta-analysis or best—based on the results of a prospective randomized trial. It has been announced previously that patients with metastases to ipsilateral lymph nodes consist of several sub-groups of different prognoses. The current paper provides with new data on late survival of patients with an “obscured” single-station N2 disease and shows that the prognosis in this group is similar to that in occult N2 metastases. The 5-year survival rate reaching almost 40% in this small group of patients is encouraging but without comparison of this survival to late results of chemoradiotherapy in this subgroup of patients recommendation of primary surgery in this subgroup does not seem justified.
False-negative results of PET-CT in the diagnosis of mediastinal nodal involvement in lung cancer patients prompts several authors to recommend performing a routine mediastinoscopy even in the absence of suspected N2 disease in CT scan or PET-CT. The most commonly accepted recommendations are as follows:
- Adenocarcinoma (except types A, B and C according to Noguchi classification) (9,12,15,16);
- Centrally located tumor (7,19,20);
- High risk of postoperative complications (e.g., pneumonectomy is considered, concomitant diseases) (19);
- Superior sulcus cancers (21);
- cN1 disease in CT scan or PET-CT (7,9,12,22);
- High diameter of the tumor (9,12) although the recommended diameter varies;
- High SUVmax of the tumor (this parameter is not standardized and varies depending on institution) (8,11,13-15,20);
- Right upper lobe (7,10); middle lobe (12) location of the primary tumor;
- Known or suspected very limited small cell lung cancer (23).
Implementation of novel non-cytotoxic agents in the systemic treatment sheds new light on the issue of adjuvant treatment, forcing surgeons and pathologists to provide better quality material for molecular tests. On the other hand, the implementation of minimally invasive surgery (VATS lobectomies and VATS segmentectomies) facilitates the use of adjuvant treatment (24,25). By shifting importance towards molecular tests, the advantage of primary surgery and surgical biopsy over fine needle aspiration is increasing. It also prompts to operate patients with locally advanced disease, including patients with minimal N2 disease, before administration of systemic treatment. Whether it will improve quality of life, time to progression and distant survival is not obvious yet and will have to be confirmed in prospective randomized trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Honguero Martínez AF, García Jiménez MD, García Vicente A, et al. Is the prognosis of occult N2 disease similar to that of positive positron emission tomography-computed tomography (PET/CT) scan single-station N2 disease in patients with non-small cell lung cancer treated by surgical resection? Rev Esp Med Nucl Imagen Mol 2017;36:350-5. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Chaft JE, Rusch V, Ginsberg MS, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol 2013;8:1084-90. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis 2014;6:S224-7. [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [Crossref] [PubMed]
- Park HK, Jeon K, Koh WJ, et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology 2010;15:1179-84. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010;37:1168-74. [Crossref] [PubMed]
- Iskender I, Kapicibasi HO, Kadioglu SZ, et al. Comparison of integrated positron emission tomography/computed tomography and mediastinoscopy in mediastinal staging of non-small cell lung cancer: analysis of 212 patients. Acta Chir Belg 2012;112:219-25. [PubMed]
- Miyasaka Y, Suzuki K, Takamochi K, et al. The maximum standardized uptake value of fluorodeoxyglucose positron emission tomography of the primary tumour is a good predictor of pathological nodal involvement in clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:83-7. [Crossref] [PubMed]
- Cho HJ, Kim SR, Kim HR, et al. Modern outcome and risk analysis of surgically resected occult N2 non-small cell lung cancer. Ann Thorac Surg 2014;97:1920-5. [Crossref] [PubMed]
- Trister AD, Pryma DA, Xanthopoulos E, et al. Prognostic value of primary tumor FDG uptake for occult mediastinal lymph node involvement in clinically N2/N3 node-negative non-small cell lung cancer. Am J Clin Oncol 2014;37:135-9. [Crossref] [PubMed]
- Lin JT, Yang XN, Zhong WZ, et al. Association of maximum standardized uptake value with occult mediastinal lymph node metastases in cN0 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;50:914-9. [Crossref] [PubMed]
- Kaseda K, Watanabe K, Asakura K, et al. Identification of false-negative and false-positive diagnoses of lymph node metastases in non-small cell lung cancer patients staged by integrated (18F-)fluorodeoxyglucose-positron emission tomography/computed tomography: A retrospective cohort study. Thorac Cancer 2016;7:473-80. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Wnuk P, Kowalewski M, Małkowski B, et al. PET-CT derived Artificial Neural Network can predict mediastinal lymph nodes metastases in Non-Small Cell Lung Cancer patients. Preliminary report and scoring model. Q J Nucl Med Mol Imaging 2014. [Epub ahead of print]. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365-9. [Crossref] [PubMed]
- Detterbeck FC. N2 Disease Discovered at Thoracotomy: Resect or Abort? In: Ferguson MK. editor. Difficult Decisions in Thoracic Surgery. London: Springer, 2011:89-103.
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Jones DR, Detterbeck FC. Pancoast tumors of the lung. Curr Opin Pulm Med 1998;4:191-7. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. [Crossref] [PubMed]
- Szczesny TJ, Szczesna A, Shepherd FA, et al. Surgical treatment of small cell lung cancer. Semin Oncol 2003;30:47-56. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]


