Staged hybrid procedure in persistent atrial fibrillation: safety, efficacy, and atrial tachyarrhythmia
The only class I recommendations in 2017 expert consensus statement on ablation of atrial fibrillation are catheter ablation of symptomatic paroxysmal atrial fibrillation refractory or intolerant to at least one antiarrhythmic medication and concomitant open surgical ablation (i.e., Cox-maze procedure) of symptomatic atrial fibrillation (1). The outcomes of catheter ablation of persistent and long-standing persistent atrial fibrillation have not been satisfactory (2-6). However, the stand-alone Cox-maze procedure of persistent atrial fibrillation without concomitant cardiac surgery is not recommended because of the risk of complications, including mortality (7,8). The minimally invasive surgical approach using video-assisted pulmonary vein ablation and exclusion of the left atrial appendage was first described in 2005 (9). In addition to potentially more durable pulmonary vein isolation, other advantages of a thoracoscopic approach include access to epicardial structures, such as the ligament of Marshall and ganglionated plexi, management of the left atrial appendage, and avoidance of damaging collateral structures, such as the phrenic nerve and esophagus (1). The minimally invasive surgical ablation not using cardiopulmonary bypass has been evolved to catch up the lesion set of conventional Cox-maze procedure. However, making a cavotricuspid isthmus line and mitral isthmus line is difficult using the epicardial ablation. The Dallas lesion set makes a trigone line instead of the traditional mitral isthmus line, and it has approached the outcomes of the Cox-maze procedure without including right atrial lesions (10,11). The hybrid approach of the two methods, which are complementary, provide an alternative approach (12-14). The Expert Consensus says that whereas persistent atrial fibrillation patients might not have been candidates for catheter ablation preoperatively, they are now ideal candidates for a “touch-up” catheter ablation after surgical ablation. The electrophysiologists will frequently find a single small break in a line, which is easily completed with a catheter resulting in a successful overall procedure.
We read the paper of Bulava et al. regarding the correlation of arrhythmia recurrence after hybrid epicardial and endocardial radiofrequency ablation for persistent atrial fibrillation with great interest (15). We would like to discuss the results regarding: (I) safety of thoracoscopic epicardial ablation; (II) timing of post-procedural endocardial electrophysiological confirmation; (III) recurrent atrial tachyarrhythmia after epicardial thoracoscopic ablation.
Safety of thoracoscopic epicardial ablation
We have a different perspective on the safety issue of epicardial thoracoscopic ablation in this article. In this report, 3.9% of conversions to sternotomy occurred because of bleeding. We also experienced two major bleeding incidences during the learning curve (16). However, until now, no bleeding complications have occurred in 296 patients. Therefore, the prevalence of major bleeding in our cohort was 0.7% (2/296). We think that bleeding requiring urgent conversion to sternotomy can be overcome following a learning period that requires about 50 cases. Phrenic nerve palsy has also posed a difficulty by causing pulmonary complications requiring surgical plication. We thought that phrenic nerve palsy was caused by heat injury during opening pericardium using unipolar electrocautery. Fortunately, we could reduce the prevalence of phrenic nerve palsy dramatically using bipolar-type electrocautery (Harmonic scalpel). In the report by de Asmundis et al., postoperative complications after epicardial surgery were reported to occur in up to 17% of total cases, but the majority of complications was minor and short-term and recovered without any sequela (17). The only complication, which is still a difficulty, is acute pericarditis. We have prescribed colchicine to all patients undergoing thoracoscopic surgery to reduce postoperative pericardial inflammation. However, in our cohort, 3% of patients suffered from chest pain due to pericarditis, requiring readmission, and the prevalence did not decrease until now.
Rationale and timing of endocardial electrophysiological confirmation
According to a recent meta-analysis, the number of atrial fibrillation-free cases increased from 85.7% to 92% when bipolar RF energy was used in thoracoscopic epicardial ablation (18). However, it has been established that transmural lesions cannot be secured in epicardial ablation using only bipolar energy. Moreover, mitral isthmus and cavo-tricuspid-isthmus ablations, which are needed in cases of persistent or longstanding persistent atrial fibrillation, are difficult to create using epicardial ablation. Thus, the synergistic effect of endocardial ablation is needed to improve rhythm outcomes in patients with persistent or long-standing persistent atrial fibrillation. The hybrid procedure, i.e., total thoracoscopic ablation together with endocardial ablation, has the potential to combine the benefits of both methods. Pison et al. reported that among 78 patients with atrial fibrillation undergoing simultaneous hybrid ablation procedures, 87% of patients were atrial fibrillation-free without antiarrhythmic drugs at a median follow-up of 24 months (19). La Meir et al. compared the hybrid epicardial and endocardial ablation in 35 patients with the epicardial ablation-only approach in 28 patients and reported that the success rates of atrial arrhythmia absence were higher in those who underwent hybrid ablation than in those who underwent epicardial ablation alone (91% versus 82%, respectively; P=0.07), particularly in those with persistent or long-standing persistent atrial fibrillation (13). The results of the current article are in line with these reports. We also agree that the efficacy of hybrid ablation seems to be very promising, especially in persistent atrial fibrillation.
The proper timing for post-procedural electrophysiological study remains controversial, and it is conducted mainly to generate additional linear lines or for complete pulmonary vein isolation, as transmural ablation lines are critical for atrial fibrillation treatment (1). The hybrid procedure can be performed in one step. For several reasons, including a lack of hybrid equipment, increased risk of postoperative bleeding during the simultaneous electrophysiological study, and collaboration with cardiologists, simultaneous hybrid procedures were not performed in our center. Despite the theoretical advantages of a simultaneous hybrid procedure, confirmation of complete pulmonary vein isolation through perfect transmurality may be limited to immediately after surgery. Magnano et al. reported that a seemingly complete transmural lesion could become an incomplete lesion over time because of the edematous or stunned state of the tissues immediately after epicardial ablation (20). Additionally, several studies showed that completeness of line and pulmonary vein isolation immediately after surgery was not directly associated with the incidence of arrhythmias over time (21). In our early practice, we performed post-procedural electrophysiological confirmations five days after surgery. The absence of atrial fibrillation at 2 years in was 93% (16). To prove the efficacy of early post-procedural electrophysiological confirmation, we have performed the randomized controlled prospective study named “Post-procedural electrophysiological confirmation upon totally thoracoscopic ablation in patients with lone persistent atrial fibrillation: A non-inferiority study” (Clinical Trial Registration: URL: http://clinicaltrials.gov; Unique identifier: NCT02392338). Based on this trial, one year postoperatively, normal sinus rhythm was observed in 89% of patients (40/45), and seven patients (16%) underwent additional catheter ablations because of residual potential in the left atrium. Seventy percent of patients undergoing thoracoscopic ablation only showed normal sinus rhythm without additional catheter ablation at postoperative 1 year (unpublished). The finding that drew our attention was that two patients who underwent concurrent cavo-tricuspid-isthmus ablation showed late cavo-tricuspid-isthmus dependent atrial flutter during the follow-up, even though bidirectional block was confirmed in those patients during the post-procedural electrophysiological confirmation before discharge. It could raise a question whether endocardial ablation of certain lines, including cavo-tricuspid-isthmus, is necessary before the window period. Accordingly, we changed the timing of endocardial procedure from an early stage (5 days after surgery) to three months after the surgery (window period), in line with a previously published paper (15). However, we perform staged hybrid procedure in patients with atrial tachyarrhythmia only despite epicardial ablation. We suggest that post-procedural electrophysiological confirmation should be considered in patients suffering from atrial arrhythmia events refractory to thoracoscopic ablation, and this approach is potentially more cost-effective compared to the hybrid approach in all patients.
Late recurrent atrial tachyarrhythmia
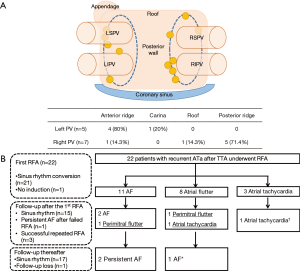
It has been known that pulmonary vein reconnection is one of the most important causes of recurrent atrial tachyarrhythmia after surgical ablation of atrial fibrillation (22,23). Previous studies described that sites commonly involved after surgical ablation using a clamp were a superior or inferior ridge of pulmonary veins, which resulted from limited energy application at the end of the two jaws (16,23,24). Among the 172 patients in our institution who had undergone thoracoscopic ablation with a minimum follow-up duration of 1 year, 24 patients showed recurrent symptomatic atrial tachyarrhythmia (unpublished). Pulmonary vein gaps were detected in 12 patients (Figure 1A). Initially, we thought that incomplete clamping around the pulmonary vein orifice could be explained by an enlarged left atrium creating a long distance between the superior and inferior pulmonary veins. However, we could not find a correlation between the incidence of additional ablation and the left atrial diameter or the left atrial volume index. Although the exit block test was performed and confirmed the pulmonary vein isolation intraoperatively, residual gaps were observed. Recently, we made a linear lesion between the superior vena cava and inferior vena cava using a cryoprobe to prevent late atrial flutter. We also made a circular lesion in the superior vena cava with a bipolar clamp, when the right atrium was significantly enlarged on the thoracoscopic view. Further study is required to verify the protective effect of these procedures against the late atrial flutter. However, after additional catheter ablation in 22 out of 24 patients, sinus rhythm was restored in 17 patients (77%, Figure 1B).
Conclusions
Regarding the hybrid ablation procedure, electrophysiological confirmation after thoracoscopic ablation might be helpful to improve not only early rhythm outcomes but also decrease the prevalence of late symptomatic atrial tachyarrhythmias. We suggest that the timing of endocardial confirmation including ablation should be after the window period (at least 3 months) following epicardial ablation.
Acknowledgements
The authors thank Joong Hyun Ahn and Keumhee C. Carriere in the Division of Biostatistics in Samsung Medical Center for statistical support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637-44. [Crossref] [PubMed]
- Mont L, Bisbal F, Hernández-Madrid A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501-7. [Crossref] [PubMed]
- Hummel J, Michaud G, Hoyt R, et al. Phased RF ablation in persistent atrial fibrillation. Heart Rhythm 2014;11:202-9. [Crossref] [PubMed]
- Bassiouny M, Saliba W, Hussein A, et al. Randomized study of persistent atrial fibrillation ablation: ablate in sinus rhythm versus ablate complex-fractionated atrial electrograms in atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9:e003596. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [Crossref] [PubMed]
- Weimar T, Schena S, Bailey MS, et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol 2012;5:8-14. [Crossref] [PubMed]
- Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797-802. [Crossref] [PubMed]
- Edgerton JR, Jackman WM, Mack MJ. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88:1655-7. [Crossref] [PubMed]
- Beller JP, Downs EA, Ailawadi G. Minimally invasive atrial fibrillation surgery: hybrid approach. Methodist Debakey Cardiovasc J 2016;12:37-40. [Crossref] [PubMed]
- Edgerton JR, Jackman WM, Mahoney C, et al. Totally thorascopic surgical ablation of persistent AF and long-standing persistent atrial fibrillation using the "Dallas" lesion set. Heart Rhythm 2009;6:S64-70. [Crossref] [PubMed]
- La Meir M, Gelsomino S, Lucà F, et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol 2013;167:1469-75. [Crossref] [PubMed]
- La Meir M. New technologies and hybrid surgery for atrial fibrillation. Rambam Maimonides Med J 2013;4:e0016. [Crossref] [PubMed]
- Bulava A, Mokracek A, Hanis J, et al. Correlates of arrhythmia recurrence after hybrid epi- and endocardial radiofrequency ablation for persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2017;10:e005273. [Crossref] [PubMed]
- On YK, Park KM, Jeong DS, et al. Electrophysiologic results after thoracoscopic ablation for chronic atrial fibrillation. Ann Thorac Surg 2015;100:1595-602; discussion 1602-3. [Crossref] [PubMed]
- de Asmundis C, Chierchia GB, Mugnai G, et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-centre experience. Europace 2017;19:58-65. [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2014;45:401-7. [Crossref] [PubMed]
- Pison L, Gelsomino S, Lucà F, et al. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg 2014;3:38-44. [PubMed]
- Magnano AR, Argenziano M, Dizon JM, et al. Mechanisms of atrial tachyarrhythmias following surgical atrial fibrillation ablation. J Cardiovasc Electrophysiol 2006;17:366-73. [Crossref] [PubMed]
- Bulava A, Mokracek A, Hanis J, et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4:e001754. [Crossref] [PubMed]
- Wazni OM, Saliba W, Fahmy T, et al. Atrial arrhythmias after surgical maze: findings during catheter ablation. J Am Coll Cardiol 2006;48:1405-9. [Crossref] [PubMed]
- Zeng Y, Cui Y, Li Y, et al. Recurrent atrial arrhythmia after minimally invasive pulmonary vein isolation for atrial fibrillation. Ann Thorac Surg 2010;90:510-5. [Crossref] [PubMed]
- Liu X, Dong J, Mavrakis HE, et al. Mechanisms of arrhythmia recurrence after video-assisted thoracoscopic surgery for the treatment of atrial fibrillation: insights from electrophysiological mapping and ablation. J Cardiovasc Electrophysiol 2009;20:1313-20. [Crossref] [PubMed]


