Noninvasive positive pressure ventilation for the treatment of acute respiratory distress syndrome following esophagectomy for esophageal cancer: a clinical comparative study
Introduction
Acute respiratory distress syndrome (ARDS) is a clinical complex mainly characterized by alveolar capillary injury and arising from various extra- and intra-pulmonary contributing factors (1-7). It is a severe stage or type of acute lung injury (ALI), clinically presenting as increased respiratory rate and respiratory distress, progressive hypoxemia, and diffuse infiltrations on chest X-ray. Thoracic surgery-related lung injury is likely to be associated with the occurrence of ARDS/ALS (8). The treatment of ARDS is a clinical challenge in thoracic surgery (9-16), and in patients with difficult expectoration, invasive mechanical ventilation requiring endotracheal intubation or tracheostomy is often necessary. However, noninvasive positive pressure ventilation (NPPV) can be an effective technique to improve gas exchange and avoid endotracheal intubation in selected patients with acute respiratory failure (ARF) due to ARDS (17-19). Although NPPV has been used successfully in the treatment of various forms of hypoxemic ARF (20), it efficacy as a treatment for ARDS and ALI remains controversial (21,22). In a meta-analysis of the use of NPPV in the treatment of ARDS/ALI from 1995 through 2009, Agarwal and his colleagues (23) found that NPPV was successful in fewer than 50% of cases. Therefore, they suggested that noninvasive ventilation (NIV) for the treatment of ARDS/ALI should be applied prudently. Nava and colleagues (24) posited that NIV should not be considered in patients with PaO2/FiO2 <200, except in those who are hemodynamically stable, while Antonelli M et al. (25) argued that NPPV should be recommended as a first-line treatment strategy because it prevented 54% of endotracheal intubations in professional centers. Thus far, there have been only few reports on the use of NPPV in patients following esophagectomy.
The present study is a retrospective analysis to determine the efficacy of NPPV in the treatment of ARDS/ALI following esophagectomy for esophageal cancer. In addition, we aimed to investigate factors related to failure of NPPV in an attempt to further define indications for NPPV in the treatment of ARDS/ALI following esophagectomy.
Methods
Patient selection
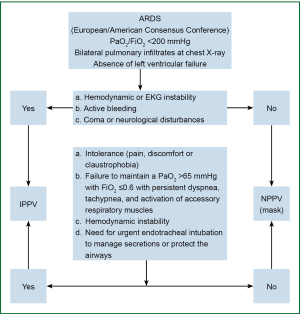
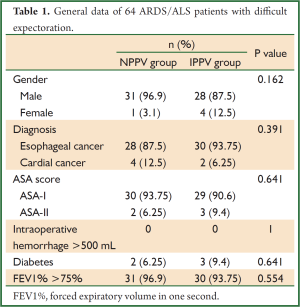
After a retrospective review of records of 1,638 patients who received surgical treatment for esophageal or cardial cancer in our hospital between January 2009 and December 2011, we found 64 who had developed ARDS/ALS and remained in the surgical ICU, and these were included in this study. The patients were classified into two groups according to the modality of mechanical ventilation: those treated with NPPV (NPPV group) and those requiring invasive positive pressure ventilation (IPPV group). Treatment of all patients followed the clinical flow chart shown in Figure 1, and the general data of these patients are shown in Table 1.
Full table
Treatments and outcome measures
Definition of ARDS/ALI: the diagnoses for all patients were based on the revised diagnostic criteria of North American and European Consensus Conference (25-27), including (I) acute onset; (II) PaO2/FiO2 ≤200 mmHg, regardless of PEEP; (III) posteroanterior chest X-ray showing patchy shadows in both lungs; and (IV) presence of hydrostatic pulmonary edema or exclusion of hydrostatic pulmonary edema due to left heart failure. The diagnosis of ALI was confirmed when PaO2/FiO2 was ≤300 mmHg in addition to the above criteria.
A cluster treatment scheme using NPPV was applied as the first treatment of choice in 48 patients with ARDS/ALI following esophagectomy. Initial exclusion criteria were hemodynamic or EKG instability, active bleeding, coma or other neurological disturbances, and need for urgent endotracheal intubation to manage secretions or protect the airway. NPPV was converted to IPPV via endotracheal intubation or tracheostomy in 16 patients because of intolerance of NPPV due to pain, discomfort, or claustrophobia; failure to maintain a PaO2 >65 mmHg with FiO2 ≤0.6 and persistent dyspnea, tachypnea, and activation of accessory respiratory muscles; hemodynamic instability; and/or need for urgent endotracheal intubation to manage secretions or protect the airways. The ventilator was a PB840 (Tyco, American).
The primary outcome variables were the length of ICU stay, 28-day survival in the ICU and in hospital admission. Secondary endpoints included the number of patients eligible for NPPV, requirement for endotracheal intubation and mechanical ventilation at any time, and risk factors associated with failure of NPPV.
Statistical analysis
Statistical analysis was performed using SPSS 13.0. In single factor analysis, measurement data were tested by t test using two independent samples, and enumeration data were tested by chi-square test. P-value of less than 0.05 was considered statistically significant.
Results
We included 64 patients (59 men and 5 women; age range, 49-83 years; mean age, 61.1±7.2 years) with ARDS/ALI following esophagectomy for esophageal cancer in our hospital between January 2009 and December 2011. There were no significant differences in gender, diagnosis, ASA score, occurrence of intraoperative hemorrhage >500 mL, diabetes, and FEV1% >75% between the NPPV group and the IPPV group. The baseline characteristics of the two groups are shown in Table 1. Thirty patients avoided intubation after application of NPPV (30/64, 48.4%), and the mean length of ICU stay in the NPPV patients was 11.5 days. Repeat bronchoscopic treatments effectively solved the problem of decreased ability of airway self-clearance, and the frequency of bronchoscopic treatments in the NPPV group averaged 1.8/day. Sixteen patients failed NPPV and were converted to IPPV. Predetermined criteria for endotracheal intubation after NPPV trial included failure to maintain PaO2 >65 mmHg with FiO2 ≤0.6 and persistent dyspnea, tachypnea, and activation of accessory respiratory muscles; need for urgent endotracheal intubation to manage copious tracheal secretions or protect the airways (i.e., coma or neurological disturbances); intolerance of NPPV (i.e., pain, discomfort, or claustrophobia); and hemodynamic instability. The average time to conversion to IPPV was 3.82±7.23 days. The 16 patients were converted to IPPV because of hemodynamic instability, active bleeding, or neurological disturbances.
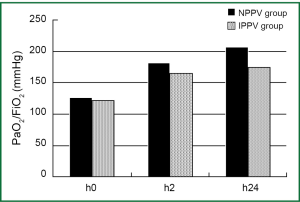
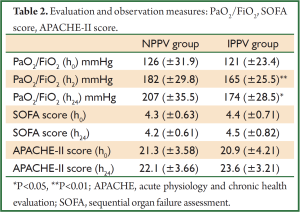
There were no significant differences in PaO2/FiO2 (NPPV 126±31.9 vs. IPPV 121±23.4), sequential organ failure assessment (SOFA) score (NPPV 4.3±0.63 vs. IPPV 4.4±0.71), or acute physiology and chronic health evaluation (APACHE-II) score (NPPV 21.3±3.58 vs. IPPV 20.9±4.21) at the time of onset between the two groups (P>0.05), nor were there significant differences in SOFA (NPPV 4.2±0.61 vs. IPPV 4.5±0.82) or APACHE-II scores (NPPV 22.1±3.66 vs. IPPV 23.6±3.21) between the two groups at 24 h after treatment (P>0.05). However, there were significant differences in PaO2/FiO2 at 2 h (NPPV 182±29.8 vs. IPPV 165±25.5, P<0.01) and 24 h (NPPV 207±35.5 vs. IPPV 174±28.5, P<0.05) after treatment between the two groups. There were no significant differences in 28-day fatality or PaO2/FiO2 at the time of onset, nor any significant differences in SOFA or APACHE-II scores at 2 and 24 h after treatment (P>0.05).
The mean length of ICU stay for patients in the NPPV group was lower than in the IPPV group (11.5 vs. 33.1, P<0.05), and the actual fatality rate in the NPPV group was significantly lower than in the IPPV group (6.25% vs. 25%, P<0.05). The 24-h PaO2/FiO2 was significantly improved in the NPPV group vs. the IPPV group (207±35.5 vs. 174±28.5 mmHg, P<0.05), and the mean number of major surgery-related complications was significantly smaller (1.25±0.58 vs. 2.13±0.81, P<0.01). When patients with ≥2 surgery-related complications were excluded, there was no significant difference in actual fatalities between the two groups (3.22%, 1/31 vs. 3.84%, 1/26; P>0.05) (Tables 2,3 and Figure 2).
Full table

Full table
Discussion
NPPV is an effective option for the treatment of ARF that can avoid endotracheal intubation or tracheotomy. According to the 2006 guidelines for the diagnosis and treatment of ALI/ARDS in China, there is not sufficient evidence to support NPPV as routine treatment for acute hypoxic respiratory failure due to ARDS/ALI, and NPPV is not suitable for patients with increased secretion, decreased ability of airway self-clearance, and a recent history of esophageal surgery. However, a retrospective meta-analysis of trials of NPPV for the treatment of ALI/ARDS between 1995 and 2009 showed that the success rate was about 50%, and suggested that NPPV could be safely be applied in appropriate cases under close supervision (23). In the present study, NPPV was successful in 30 of 32 patients in the NPPV group and the mean length of ICU stay was 11.5 days. Our clinical observations indicated that multiple repeat bronchoscopic treatments could effectively solve the problem of decreased ability of airway self-clearance, with the patients in the NPPV group averaging 1.8 bronchoscopic treatments daily. Thus, esophageal surgery may not be an absolute contraindication for NPPV. Considering that 16 of 32 patients in our series who required IPPV for ARDS after esophagectomy had also received NPPV treatment previously, the overall success rate of NPPV was 64.6%, and the NPPV success rate in ARDS was 48.4%. It could therefore be concluded that NPPV is a good treatment option in appropriate cases and can minimize trauma to these patients.
Studies by Antonelli et al. (25) and Yoshiida et al. (26) showed that there was no significant difference in PaO2/FiO2 between NPPV success groups and NPPV failure groups in the initial stage. However, as PaO2/FiO2 improved continuously after treatment in the NPPV success group, it was considered an independent factor for predicting failure of NPPV in the treatment of ALI (26). Antonelli et al. (25) also proposed that PaO2/FiO2 OI ≤175 at 1 h after NPPV was an independent factor for predicting failure of NPPV for the treatment of ALI. Likewise, we found no significant difference in PaO2/FiO2 between IPPV and NPPV groups in the initial stage, but significant differences at 2 (P<0.01) and 24 h (P<0.05) between the two groups, indicating that PaO2/FiO2 might be a predictor for success or failure of NPPV treatment. Intra-group comparison in the present study (NPPV 182±29.8 vs. IPPV 165±25.5) suggested that PaO2/FiO2 >180 at 2 h after treatment is a feasible indicator for continuation with NPPV treatment. Of course, this conclusion needs to be confirmed by more data.
After patients with ≥2 major surgery-related complications were excluded, there was no significant differences in actual fatalities between the two groups (3.22%, 1/31 vs. 4%, 1/25; P>0.05). Thus, IPPV may be the best first choice for ARDS patients with ≥2 major surgery-related complications after esophageal surgery, and in such cases, early oral intubation or tracheostomy is required.
In the NPPV group, the 24-h PaO2/FiO2 was significantly improved as compared with the IPPV group (207±35.5 vs. 174±28.5 mmHg, P<0.05), and the mean number of major surgery-related complications was significantly smaller (NPPV 1.25±0.58 vs. IPPV 2.13±0.81, P<0.01). We speculated that the pathological conditions might have been a factor in the relatively larger number of patients with major surgery-related complications in the IPPV group.
In summary, NPPV can be an effective option for the treatment of ARDS/ALI following esophagectomy for esophageal cancer in select patients, with recurrent bronchoscopic therapy to address the problem of decreased ability of airway self-clearance. However, in patients with two or more severe postoperative complications, including acute renal failure and cardiac arrest, and those with PaO2/FiO2 <180 at 2 h after NPPV treatment, invasive mechanical ventilation is required.
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant Numbers: 81200038, 81170491) and Foundation for Young Talents of Guangzhou Education Bureau (Grant Number: 10A152).
Disclosure: The authors declare no conflict of interest.
References
- Zhang Z. Protective ventilation for patients without acute respiratory distress syndrome. JAMA 2013;309:654. [PubMed]
- Wang W, Zhou Y, Su Z, et al. Acute respiratory distress syndrome in one patient with gout complicated by severe pulmonary tuberculosis: report of one case and literature review. J Thorac Dis 2013;5:E50-2. [PubMed]
- Soma K. Acute respiratory distress syndrome and pneumonia. Masui 2013;62:547-56. [PubMed]
- Solsona Durán JF, Basas Satorras M, Zapatero Ferrándiz A, et al. Acute respiratory distress syndrome criteria. Med Intensiva 2013;37:124. [PubMed]
- Sigurdsson MI, Sigvaldason K, Gunnarsson TS, et al. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand 2013;57:37-45. [PubMed]
- Laffey JG, Talmor D. Predicting the development of acute respiratory distress syndrome: searching for the “Troponin of ARDS”. Am J Respir Crit Care Med 2013;187:671-2. [PubMed]
- Ichikado K. “The Berlin definition” and clinical significance of high-resolution CT (HRCT) imaging in acute respiratory distress syndrome. Masui 2013;62:522-31. [PubMed]
- Kometani T, Okamoto T, Yoshida S, et al. Acute respiratory distress syndrome after pulmonary resection. Gen Thorac Cardiovasc Surg 2013;61:504-12. [PubMed]
- Wise MP, Saayman AG, Gillies MA. High-frequency oscillatory ventilation and acute respiratory distress syndrome: at the crossroads? Thorax 2013;68:406-8. [PubMed]
- Tanaka R. Strategy of mechanical ventilation for acute respiratory distress syndrome. Masui 2013;62:532-40. [PubMed]
- Talmor DS, Loring SH. Esophageal pressures in acute respiratory distress syndrome: how should we interpret and use them? Crit Care Med 2013;41:e1. [PubMed]
- Schultz MJ, Juffermans NP, Matthay MA. From protective ventilation to super-protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:963-5. [PubMed]
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2013;2:CD003844. [PubMed]
- Mauri T, Bellani G, Confalonieri A, et al. Topographic distribution of tidal ventilation in acute respiratory distress syndrome: effects of positive end-expiratory pressure and pressure support. Crit Care Med 2013;41:1664-73. [PubMed]
- Li XH, Li FX, Xiao ZL. Therapeutic strategies for severe acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25:186-9. [PubMed]
- Fuller BM, Mohr NM, Drewry AM, et al. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care 2013;17:R11. [Epub ahead of print]. [PubMed]
- Wang YM, Tao YH. Mechanical ventilation in acute respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2013;15:496-501. [PubMed]
- Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013;368:806-13. [PubMed]
- Sinha P, Sanders RD, Soni N, et al. Acute respiratory distress syndrome: the prognostic value of ventilatory ratio--a simple bedside tool to monitor ventilatory efficiency. Am J Respir Crit Care Med 2013;187:1150-3. [PubMed]
- Hess DR. The evidence for noninvasive positive-pressure ventilation in the care of patients in acute respiratory failure: a systematic review of the literature. Respir Care 2004;49:810-29. [PubMed]
- Sud S, Sud M, Friedrich JO, et al. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2013;2:CD004085. [PubMed]
- Fuller BM, Mohr NM, Carpenter CR. Protective ventilation for patients without acute respiratory distress syndrome. JAMA 2013;309:654-5. [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care 2010;55:1653-60. [PubMed]
- Nava S, Schreiber A, Domenighetti G. Noninvasive ventilation for patients with acute lung injury or acute respiratory distress syndrome. Respir Care 2011;56:1583-8. [PubMed]
- Antonelli M, Azoulay E, Bonten M, et al. Year in review in Intensive Care Medicine 2009. Part III: mechanical ventilation, acute lung injury and respiratory distress syndrome, pediatrics, ethics, and miscellanea. Intensive Care Med 2010;36:567-84. [PubMed]
- Yoshida Y, Takeda S, Akada S, et al. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth 2008;22:201-6. [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [PubMed]