Marfan syndrome with pneumothorax: case report and review of literatures
Introduction
Marfan syndrome is a multisystem connective tissue disorder of autosomal dominant inheritance, involving manifestations of the cardiovascular, skeletal, and ocular systems (1,2). The incidence of Marfan syndrome is approximately 2–3 in every 10,000 individuals, and pulmonary involvement occurs much less frequently. Previously, few publications described spontaneous pneumothorax in Marfan syndrome (3,4) and not until recently, the association between pneumothorax and Marfan syndrome was further explored by Karpman et al., the incidence ranged between 4.8% and 11% (5). We here present a case with spontaneous pneumothorax as an initial diagnosis of Marfan syndrome.
Case presentation
A 28-year-old woman was admitted to the emergency with hours of sudden onset, progressive shortness of breath and right-sided chest pain. She received chest computed tomography (CT) which suggested hydropneumothorax in right lung with the lung collapsed by 90%. A chest tube was inserted into the thoracic cavity to assure the expansion of the collapsed lung. Due to the chest deformity, the drainage was ineffective and the patient was admitted to our hospital for further treatment.
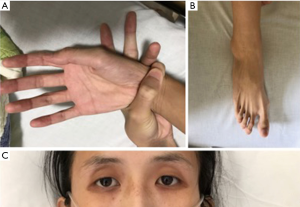
Historically, the patient had severe scoliosis and underwent orthopedic surgery 14 years ago. There was no history of previous trauma, smoking or illicit drug use. Her mother had abdominal aortic aneurysm. On physical examination, she presented severe scoliosis with decreased breath sound on the right side of chest, and long limbs with arachnodactyly (Figure 1).
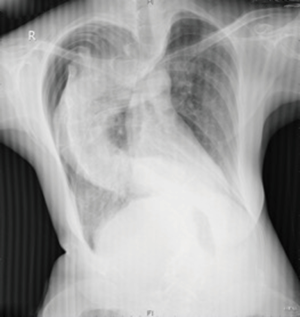
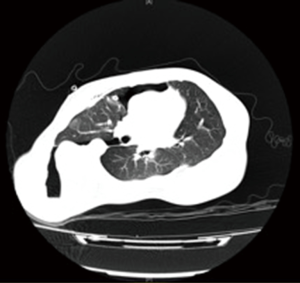
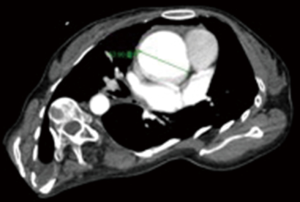
Chest radiography (Figure 2) and another CT (Figure 3) were performed after admission, which showed right-sided pneumothorax and right lung bullae, together with severe scoliosis. Based on the suspicion of Marfan syndrome, cardiac ultrasound and aortic computer tomographic angiography (CTA) (Figure 4) were performed, which showed aneurysm-like aortic root dilation at 52 mm with mild to moderate aortic regurgitation, mild mitral valve prolapses with mild to moderate mitral valve regurgitation.
According to the 2010 Revised Ghent Nosology for Marfan syndrome (6), the patient was diagnosed as Marfan syndrome and she presented the following situations: (I) suspected family history; (II) aneurysm-like aortic root dilation at 52 mm in diameter; (III) severe scoliosis (>20°); (IV) characteristic facial appearance: enophthalmos, dolichocephaly, underdeveloped cheekbones; (V) severe myopia >3 diopters; (VI) spontaneous pneumothorax; (VII) arachnodactyly; (VIII) mild to moderate mitral valve prolapse; (IX) positive wrist sign (Figure 1A).
Surgical repair of the thoracic aorta (David operation) and bullectomy were undertaken as treatments for severe aortic root dilation (7,8) and progressive pneumothorax after Marfan syndrome was diagnosed. Intraoperative transesophageal echocardiography showed dilations in aortic sinus and ascending aorta. There was mild to moderate aortic valve regurgitation without thickening or calcification of aortic valves. With the patient positioned supine, operative exposure was obtained via median sternotomy. The aneurysm-like aortic sinus was exposed and there were dilations of aortic sinus at 55 mm and of ascending aorta at 28 mm in diameter. Intracardiac exploratory operation showed aneurysm-like aortic sinus dilation at 55 mm in diameter without thickening of aortic valves or restriction of valve movements. The patient underwent David I operation and the aortic sinus was replaced with an artificial tube graft of 32 mm in diameter. Via opening the right mediastinal pleura, the exploration of the right chest cavity was achieved and the bullectomy was performed by using endoscopic staplers.
The patient underwent an event-free recovery and was discharged 20 days post-operatively. Chest X-ray showed less pneumothorax after the surgery.
Discussion
The case introduced a patient with undiagnosed Marfan syndrome who was admitted to hospital due to spontaneous pneumothorax as an initial symptom. The diagnosis of Marfan syndrome was based on her marfanoid habitus, imaging characteristics and the history of severe scoliosis and myopia (6). After the diagnosis was confirmed, David I operation and bullectomy were performed.
Before this onset of pneumothorax, the cardiac function of the patient was in compensatory period (NYHA I). Untypical manifestations as pneumothorax and scoliosis without apparent cardiovascular and ocular systematic appearances may contribute mainly to the undiagnosing of Marfan syndrome of this patient. Prevalence of pneumothorax in patients with Marfan syndrome was reported between 5% and 11% (3-5). Approximately, 16% of patients with Marfan syndrome have pulmonary symptoms (3), and pulmonary involvements may contribute to 10% of death in patients with Marfan syndrome (9). Pneumothorax usually occurred after the diagnosis in most of the cases of Marfan syndrome. Cases with pneumothorax as initial diagnosis of Marfan syndrome are uncommon, and even less common in adults. Rocha et al. (10) study in 2008 described a 14-year-old boy and Viveiro et al. (11) study in 2013 described a 16-year-old young individual, both of whom were previously healthy and had developed recurrent spontaneous pneumothorax as initial symptoms of Marfan Syndrome.
Although pulmonary involvements are not generally considered a main feature of Marfan syndrome, many patients have a degree of underlying pulmonary pathology. Patients with Marfan syndrome usually harbor mutations involving the gene FBN1 encoding the connective tissue protein fibrillin-1 (12). Fibrillin-1 is an important matrix component of both elastic and nonelastic tissues. The pulmonary changes include widespread or patchy cystic changes, emphysema, and spontaneous pneumothorax; focal pneumonia or bronchiectasis, bullae, congenital pulmonary malformations (particularly middle lobe hypoplasia), and apical fibrosis (13). The increased risk of pneumothorax can be attributed to the presence of apical blebs, bullae, abnormal connective tissue constituents in the lung parenchyma or increased mechanical stresses in the lung apices due to the tall body habitus (5).
Early diagnosis based on untypical symptoms of Marfan syndrome like pneumothorax and bullae helped in the early evaluation of cardiovascular system and early managements of the disease, which proved to increase life expectancy of patients with Marfan syndrome markedly. Sudden death and emergency operations of undiagnosed and untreated Marfan syndrome are frequently associated with aortic dissection. Aortic dissection is the main cause of death in patients with Marfan syndrome, approximately at 46% (9). Dilatation of the aorta is found in approximately 60% to 80% of adult patients (14). Early management of fatal changes like prevention of aortic dissection and progression of aorta dilatation can markedly increase life expectancy and quality. Prophylactic aortic surgery has been recommended when the aortic root diameter is greater than 5 cm and it is believed that 10-year postoperative survival of patients with Marfan syndrome was improved to 98.9% in those without dissection and 83.6% in those with dissection (1,7-9,15,16).
In patients with Marfan syndrome but without pulmonary symptoms, particular attention is suggested to the prevention of pneumothorax and other lung diseases (17,18). The Corsico’s study in 2014 reported that pneumothorax occurred in 14% of the patients with no previous respiratory symptoms and only 37% of patients had normal lung function (19). Especially in the patients with moderate-to-severe pectus excavatum or scoliosis, distinct reductions in total lung capacity can be found as well as in FVC and FEV1 (17).
Progress in the past decades has led to an improved understanding of the cause, pathophysiology, clinical manifestations, and treatments of Marfan syndrome. Early diagnosis of Marfan syndrome related to untypical manifestations such as pneumothorax and other pulmonary symptoms should be considered in the differential diagnosis.
Acknowledgements
Funding: This study was supported by National Nature Science Foundation for Young Scholars of China (grant no. 81400681).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965-76. [Crossref] [PubMed]
- Jang SY, Seo SR, Park SW, et al. The Prevalence of Marfan Syndrome in Korea. J Korean Med Sci 2017;32:576-80. [Crossref] [PubMed]
- Wood JR, Bellamy D, Child AH, et al. Pulmonary disease in patients with Marfan syndrome. Thorax 1984;39:780-4. [Crossref] [PubMed]
- Hall JR, Pyeritz RE, Dudgeon DL, et al. Pneumothorax in the Marfan syndrome: prevalence and therapy. Ann Thorac Surg 1984;37:500-4. [Crossref] [PubMed]
- Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration 2011;82:219-24. [Crossref] [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8. [Crossref] [PubMed]
- Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation 2005;111:e150-7. [Crossref] [PubMed]
- Chiu HH, Wu MH, Chen HC, et al. Epidemiological profile of Marfan syndrome in a general population: a national database study. Mayo Clin Proc 2014;89:34-42. [Crossref] [PubMed]
- Rocha S, Pereira L, Barreto C. Spontaneous pneumothorax - a clue to another diagnosis. Rev Port Pneumol 2008;14:699-704. [Crossref] [PubMed]
- Viveiro C, Rocha P, Carvalho C, et al. Spontaneous pneumothorax as manifestation of Marfan syndrome. BMJ Case Rep 2013;2013:bcr2013201697. [Crossref] [PubMed]
- Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991;352:337-9. [Crossref] [PubMed]
- Dyhdalo K, Farver C. Pulmonary histologic changes in Marfan syndrome: a case series and literature review. Am J Clin Pathol 2011;136:857-63. [Crossref] [PubMed]
- Roman MJ, Devereux RB, Kramer-Fox R, et al. Comparison of cardiovascular and skeletal features of primary mitral valve prolapse and Marfan syndrome. Am J Cardiol 1989;63:317-21. [Crossref] [PubMed]
- Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med 1999;340:1307-13. [Crossref] [PubMed]
- Pearson GD, Devereux R, Loeys B, et al. Report of the National Heart, Lung, and Blood Institute and National Marfan Foundation Working Group on research in Marfan syndrome and related disorders. Circulation 2008;118:785-91. [Crossref] [PubMed]
- Streeten EA, Murphy EA, Pyeritz RE. Pulmonary function in the Marfan syndrome. Chest 1987;91:408-12. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Corsico AG, Grosso A, Tripon B, et al. Pulmonary involvement in patients with Marfan Syndrome. Panminerva Med 2014;56:177-82. [PubMed]