Multidisciplinary approach to chest wall resection and reconstruction for chest wall tumors, a single center experience
Introduction
Chest wall resection and reconstruction (CWRR) is quite challenging due to evolving issues in technologies and techniques.
For neoplastic disease it can be carried out in three different clinical settings: (I) direct invasion from non-small cell lung cancer (NSCLC); (II) primary chest wall tumors; (III) chest wall metastasis or direct infiltration from other malignancies (1).
The treatment of NSCLC invading the chest wall does not generally entail technical problems, as resection involves only some ribs. Whereas, in primary malignant chest wall tumors, wide en-bloc resection is the key for a successful management (2), but the extent of the required demolition may be an obstacle for the curative resection, because an adequate reconstruction may difficult to guarantee. For metastatic tumors, curative surgery is seldom performed and can be considered only as part of a multidisciplinary treatment program for selected patients with differentiated thyroid cancer (1). An optimal surgical approach for chest wall tumors needs to consider in its analysis three different issues: the extension of resection, restoration of skeletal stability and soft tissue coverage (3,4). These issues require surgical expertise and full cooperation between thoracic and plastic surgeons in patients undergoing extensive full-thickness chest wall demolition (5). Surgery should be tailored on patient and on disease and the multidisciplinary approach is often the only way to obtain satisfactory results in terms of oncological, functional and aesthetic outcome.
The aim of this single-center retrospective study was to evaluate the impact of the multidisciplinary approach, including thoracic and plastic surgeons, on the management of patients with chest wall tumors undergoing extensive resection over a limited follow-up.
Methods
Ethic statement
According to National and institutional regulations patients have signed informed consent for data review. Institutional ethic board allowed for retrospective data analysis and publication for scientific purposes.
Seven cases of CWRR treated both by thoracic and reconstructive surgeons were performed between 2014 and 2016 at The University of Perugia Medical School: three sternal allograft transplantations and four CWRR reconstructions using other techniques.
Patients data are described in Table 1. The series included six males and one female, ages ranging from 40 to 78 years (median: 55 years; mean: 60.1 years). All patients had a palpable chest tumor and only four patients complained of thoracic pain prior to surgery. All surgical plans were tailored for each patient’s overall characteristics as well as type, dimension and extension of tumor. These plans were drafted by the practicing plastic and thoracic surgeons, after agreeing on the resection and reconstruction approach.
Full table
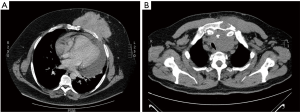
All cases were discussed in a multidisciplinary meeting with radiologists, pathologists, oncologists and radiation oncologists all in attendance. The diagnostic workup included contrast computed tomography (CT) (Figure 1), magnetic resonance imaging and positron emission tomography. Preoperative diagnosis was obtained in only three patients via core needle biopsy. Patients having chondromatous lesions underwent surgery without preoperative tissue diagnosis due to the difficulty in performing differential diagnosis between a benign tumor and a corresponding well-differentiated malignant tumor. Cardiopulmonary reserve was carefully assessed: spirometry, hemogasanalysis, electrocardiography and echocardiography for all patients; noninvasive methods for coronary flow reserve assessment for three patients.
All patients underwent surgery with curative intent after tailored surgical planning. Each wide en-bloc radical excision had circumferential radical margins of at least 4–5 cm. Reconstruction programs involved the restoration of the skeletal support, by the thoracic team, and the soft tissue reconstruction, by the plastic surgery team.
All patients underwent clinical evaluation at one month after surgery. Clinical, surgical and oncological records were retrospectively reviewed and all patients were followed-up for at least 6 months (Table 2).
Full table
This was a retrospective study and all eligible patients had to give their informed consent prior to inclusion.
Results
Duration of surgery was between 4 and 9 hours (median: 6 hours; mean: 6.2 hours). Macroscopically free-margins were achieved in all patients. All patients were transferred intubated to our intensive care unit for 24 hours after the operation for monitoring.
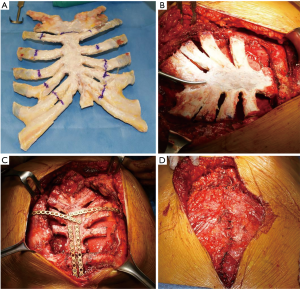
When a wide sternal resection was scheduled (patients #1, 2 and 3) the local tissue bank (Tissue bank, Treviso, Italy) provided a suitable cryopreserved sternal graft within 15–30 days of the request. The sternal allograft was completely defrosted the day before surgery. Reconstruction was performed after graft tailoring on the removed specimen (Figure 2A,B). The neo-sternum was fixed to the native manubrium and ribs with titanium bars (Stratos system, Stratos, MedXpert GmbH, Germany) (Figure 2C). In patient #1, resection also involved the sternal manubrium, the proximal part of both clavicles and the anterior bilateral rib arches. Being so, the graft was fixed with titanium bars also on the native clavicular stumps and the sternal allografts were covered and protected by a pectoralis major flap (Figure 2D).
One patient (patient #3) required a pre-operative embolization of the tumor, which was widely vascularised at the contrast CT scan. Over the course of angiography, this tumor showed several vessels arising from the ipsilateral internal mammary artery. Given this, embolization of the mass was performed 24 hours before surgery (Figure 3).

Two patients underwent resection of the distal sternum, anterior rib arches and soft tissues (including mastectomy) for primary bone tumor. Patient #4, underwent reconstruction with Marlex mesh and latissimus dorsi flap coverage, as well as a new areola implant. In patient #5, a Dual-Mesh prosthesis and titanium bar were used that were both covered by a latissimus dorsi myocutaneous flap (Figure 4). The cryopreserved sternal allograft was deemed not suitable for patients #5 and 6, as their chest wall defects involved their distal sternal bodies.
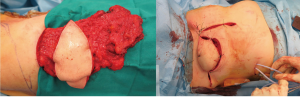
Patient #7, who had a bulky chest wall chondrosarcoma involving the left costal arch, underwent wide en-bloc radical excision of the tumor which included the lower 5 ribs, diaphragm, soft tissues and skin. Reconstruction was carried out by combining the anterior phrenoplasty, Dual Mesh prosthesis and the ipsilateral rectus abdominis flap.
In all seven cases, pre-operative planning was respected intraoperatively.
No paradox, flail chest or ventilation issues were encountered. Two early postoperative complications were observed in two patients who had undergone sternal transplantation: mild acute renal failure, on days two and three after the mass embolization, which was resolved without dialysis (patient #3); minimal skin necrosis of the myocutaneous flap at the level of the proximal corner of the skin suture (patient #5).
Histological analyses showed: one micro invasive liposarcoma, one desmoid tumour, four chondrosarcomas and one metastasis from a papillary tumor of the thyroid. The invasive liposarcoma showed micro invasion of surrounding tissues and was the only resection which could not be considered radical from an oncological point of view.
Follow-up ranged from 6 to 23 months (median: 13 months; mean: 12 months). No local relapses were recorded. Patient #5 who had undergone R1 resection, later underwent postoperative adjuvant radiotherapy and was disease free 7 months after surgery. Patient #1 who had been treated for sternal metastasis, had unmodified pulmonary metastases, when compared to preoperatively contrast CT-scan results, at 14 months after surgery.
A late complication was recorded in patient #2: CT scan taken 11 months after surgery showed an asymptomatic fracture of the sternal allograft at the level of the titanium bar fixation point. The patient reported having had a mild trauma one month earlier. Twenty-three months after surgery, the patient was totally asymptomatic, in good general condition and his graft was still firmly in place with neither paradox nor breathing issues.
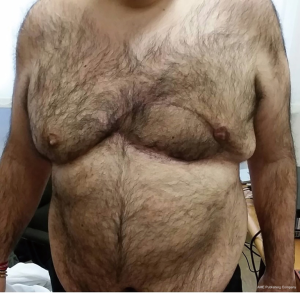
Finally, with only thin and uncontaminated scars remaining, the aesthetic results were deemed acceptable (Figure 5).
Discussion
The aim of this single-centre retrospective study was to evaluate the impact of the multidisciplinary approach on both functional and aesthetical outcomes, as well as prognosis in patients with neoplastic diseases of the chest wall.
Wide en-bloc resection, with 4–5 cm of free resection margins, has been reported to be an effective management approach for primary malignant chest wall tumors (1). However, any resulting defect may pose problems for reconstruction, especially when large amounts of bony thorax, skin and/or soft tissue are included in the resection. Any anticipated reconstruction issues should not influence the radical resection (5). For these reasons cooperation between thoracic and plastic surgeons is frequently required to achieve a quality management of these tumors (5-7). In selected, highly vascularised tumors, pre-operative embolization could be helpful in shrinking the mass volume of the tumor and limiting any intraoperative bleeding (8).
As is well known, size and location of the surgical defect play roles in patient outcome (1). A key factor that favors good outcome is a well-planned reconstruction. Anterior chest wall resection has always been considered a challenging intervention in CWRR, because of the instability of that thoracic region and also due to the recurrent exposition of internal viscera. In fact, in anterior CWRR, prosthetic reconstruction has been proven to achieve low rates of morbidity, mortality and complications, especially in patients with low respiratory function (3,4).
Ideal materials do not exist for prostheses, as all materials, absorbable or not, rigid or flexible, have both advantages and disadvantages (9-12). Titanium bars are high in strength with low weight, biocompatible, easily pliable and adaptable. However, titanium bar failure after implantation has been described (13) and here we report on an issue to this regard. Nevertheless, prosthetic materials are widely utilized for CWRR and are essential for repairing wide defects, restoring skeletal stability, maintaining chest volume and protecting internal viscera. The choice of material remains primarily surgeon-dependent.
Helpful techniques in chest reconstruction include phrenoplasty and omentoplasty. The former was developed for the treatment of tuberculosis and is sometimes used for reconstruction when the last ribs are demolished, so the thoracic defect is transformed into an abdominal defect, avoiding thoracic and ventilation complications (1). Whereas the latter is used for infected or irradiated wounds; in these cases, a well-vascularised flap is preferable for eliminating infections and has been proven to be effective for treating deep sternal contaminations (14,15). In our series, we report on three cases of sternal allograft transplantation; a technique considered experimental up until a few years ago. Sternal allograft transplantation is biocompatible, does not favor the onset of infectious diseases, can receive other synthetic prostheses (titanium bars) and can be tailored on the defect (16-18). Furthermore, the bone allograft is subjected to the same incorporation process already described for the bone heterograft (19). In order to promote graft incorporation, the bone allograft has to be covered with highly vascularised soft tissues. In our series, pectoralis major flaps resulted being suitable.
The extent of the resection must be pre-determined, so to evaluate if a multidisciplinary approach involving plastic and thoracic surgeons may be required. When deemed necessary, the best soft tissue reconstruction is always the simplest possible. Whereas, the coverage of large full-thickness chest wall defects is not always a simple procedure, especially if a very large skin resection is scheduled (1).
The definitive coverage of full thickness defects requires experienced surgeons and good planning. Whatever the material utilized for skeletal reconstruction, a complete coverage with soft tissues is essential for avoiding skin and subcutaneous tissue closures above the prosthesis (1). In the case of wide defects, covering the prosthesis with a vascularised flap has been reported to lead to better functional and aesthetic outcomes. Similarly, when the skeletal defect is not too wide, the reconstruction of bony thorax could be avoided and the skeletal stability could be restored only using soft tissues reconstruction. The most used flaps in CWRR include the latissimus dorsi, pectoralis major, rectus abdominis, trapezius and external oblique muscular ones. Here, local flaps are preferable to distant flaps. The choice of the flap depends on the type of reconstruction, size and location of the defect, the width of the resection and any previous radiotherapy and/or local surgery. These flaps can be used individually or in combination (6,7). In our experience to date, pectoralis major flaps have been our first choice for defects located in the upper sternal region while rectus abdominis flaps have been used to repair lower sternal defects. We have used latissimus dorsi flaps to cover the largest thoracectomies involving soft tissues and its regional blood supply has allowed for the obtainment of the most suitable myocutaneous flap.
Conclusions
One-time procedures can reduce the rates of morbidities and complications in patients requiring CWRR. In order to perform CWRR, the width and depth of any defect needs to be estimated and planned in a multidisciplinary approach that includes plastic and thoracic surgeons, especially if a large resection of the skin is deemed necessary. Specifically, this multidisciplinary approach entails a pre-operative program where the surgical skills are integrated to obtain a single procedure having pre-determined phases, in order to facilitate the execution of the intervention and reduce any complications. The decisions on the choices of reconstructive materials and flaps need to be agreed upon between the two surgical groups, based on surgical experience and confidence with techniques and materials.
In our series, this multidisciplinary approach led to the performance of radical CWRR, a one-time procedure, resulting in acceptable postoperative morbidity and mortality rates, as well as acceptable functional and aesthetic results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: According to National and institutional regulations patients have signed informed consent for data review. Institutional ethic board allowed for retrospective data analysis and publication for scientific purposes.
References
- Puma F, Vannucci J. Chest wall resection/reconstruction for tumors. In: Fischer JE, editor. Master Techniques in Surgery. Thorac Surg Philadelphia: W. Kluwer; 2015:312-58.
- Facciolo F, Cardillo G, Lopergolo M, et al. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001;121:649-56. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Dabandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- Cohen M, Ramasastry S. Reconstruction of complex chest wall defects. Am J Surg 1996;172:35-40. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Chest wall reconstruction. Experience with 100 consecutive patients. Ann Surg 1984;199:725-32. [Crossref] [PubMed]
- Puma F, Cardini CL, Passalacqua G, et al. Preoperative embolization in surgical management of giant thoracic sarcomas. Eur J Cardiothorac Surg 2008;33:127-9. [Crossref] [PubMed]
- Puma F, Ragusa M, Daddi G. Chest wall stabilization with synthetic reabsorbable material. Ann Thorac Surg 1992;53:408-11. [Crossref] [PubMed]
- Puma F, Ragusa M, Santoprete S, et al. Chest wall stabilization with synthetic reabsorbable material. Updated in 1999. Ann Thorac Surg 1999;67:1823-4. [Crossref] [PubMed]
- Rocco G. Overview on current and future materials for chest wall reconstruction. Thorac Surg Clin 2010;20:559-62. [Crossref] [PubMed]
- Azoury SC, Grimm JC, Tuffaha SH, et al. Chest Wall Reconstruction: Evolution over a decade and experience with a novel technique for complex defects. Ann Plast Surg 2016;76:231-7. [Crossref] [PubMed]
- Berthet JP, Caro AB, Solovei L, et al. Titanium Implant Failure After Chest Wall Osteosynthesis. Ann Thorac Surg 2015;99:1945-52. [Crossref] [PubMed]
- Puma F, Fedeli C, Ottavi P, et al. Laparoscopic omental flap for the treatment of major sternal wound infection after cardiac surgery. J Thorac Cardiovasc Surg 2003;126:1998-2002. [Crossref] [PubMed]
- Tassi V, Ceccarelli S, Vannucci J, et al. Mediastinitis and sternal prosthesis infection successfully treated by minimally invasive omental flap transposition. J Cardiothorac Surg 2013;8:30. [Crossref] [PubMed]
- Dell’Amore A, Cassanelli N, Dolci G, et al. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact Cardiovasc Thorac Surg 2012;15:944-7. [Crossref] [PubMed]
- Nosotti M, Rosso L, Mendogni P, et al. Sternal reconstruction for unusual chondrosarcoma: innovative technique. J Cardiothorac Surg 2012;7:40. [Crossref] [PubMed]
- Marulli G, Dell'amore A, Calabrese F, et al. Safety and effectiveness of cadaveric allograft sternochondral replacement after sternectomy: a new tool for the reconstruction of anterior chest wall. Ann Thorac Surg 2017;103:898-905. [Crossref] [PubMed]
- Puma F, Avenia N, Ricci F, et al. Bone heterograft for chest wall reconstruction after sternal resection. Ann Thorac Surg 1996;61:525-9. [Crossref] [PubMed]


