New insights into stage and prognosis in small cell lung cancer: an analysis of 968 cases
Introduction
Due to its incidence and mortality worldwide, small cell lung cancer (SCLC) is a notable healthcare issue (1). SCLC accounts for 10–15% of all lung cancers (2). In 2011, according to the Surveillance, Epidemiology, and End Results program (SEER), 5-year survival rate was 6.5% for patients with SCLC and 22.1% for those with non-small cell lung cancer (NSCLC) (3). This poor prognosis reflects the rapid growth of SCLC, its propensity for spread to lymph nodes and distant organs, and the higher proportion of advanced diseases at diagnosis (2).
Despite its importance, as evidenced by a simple 5-year research of Internet (PubMed) performed on 10 October 2015, which found 1,421 articles for SCLC (MeSH) vs. 12,253 articles for NSCLC (MeSH), SCLC is poorly studied. The management of SCLC and survival rates has not improved since the first reports of the disease by Bernard in 1926, and the primary forms of therapy in the 1960s–1980s (with the advances in staging and the advent of chemotherapy and radiation therapy) (4). Combination chemotherapy (usually platinum-based plus etoposide or irinotecan) remains the first-line therapy for metastatic SCLC and for non-metastatic disease in association with early concurrent thoracic radiotherapy (1).
The French College of General Hospital Respiratory Physicians (CPHG) has conducted two prospective multicentre epidemiological studies at a 10-year interval: KBP-2000-CPHG and KBP-2010-CPHG (5-9). These studies included all consecutive new cases of primary lung cancer histologically or cytologically proven in 2000 or 2010 and followed in the respiratory department of non-academic hospitals. More than 900 of the 5,667 and 7,051 patients included in KBP-2000-CPHG and KBP-2010-CPHG cohorts had a SCLC (8). The large KBP-2010-CPHG cohorts allow descriptive statistics and outcome assessment for SCLC and NSCLC separately. The similarity of the design of both studies allows comparison between the two SCLC cohorts over a 10-year period.
We therefore present the characteristics and 1-year mortality of the 968 new cases of SCLC diagnosed in 2010 and compare results with those obtained for the 6,083 new cases of NSCLC diagnosed in 2010 and those obtained for the 948 cases of SCLC reported in 2000.
Methods
The study protocols were approved by French Information Technology and Freedoms Commission (CNIL) on 02 August, 2000 (No. 900019) and 11 January, 2010 (No. 909479). The KBP-2010-CPHG protocol was also approved by the advisory committee on research information processing in the health field (CCTIRS) on 19 November, 2009. The ethics committee of the French Society of Pneumology confirmed the observational nature of the study on 23 April 2010 (No. 2010–008). All patients were duly informed of the study objectives and requirements and gave oral consent before inclusion.
The members of the CPHG which gathers the chest physicians of the respiratory departments of the French non-academic hospitals were contacted. Those agreeing to participate became study investigators and their departments study centres. Participation in one study was independent of participation in the other study. Each investigator was to include all consecutive patients aged over 18 years with primary lung cancer histologically or cytologically proven between 01 January and 31 December (date of sampling) and managed in his/her study centre. For each included patient, the investigator filled out an anonymous questionnaire comprising items on age, sex, smoking, performance status (PS), histologic tumour type, tumour stage (6th version for KBP-2000-CPHG and 7th version for KBP-2010-CPHG), and first-line (initial) therapy (KBP-2010-CPHG, only). A steering committee assessed study completeness by checking the regularity of inclusions throughout the year for all centres individually, and taken together, and the coherence of the data between 2000 and 2010 for centres which participated in both studies. Clinical research associates checked the completion of the questionnaires and contributed to the completeness of the recruitment (5,7,8).
The population was described in terms of the questionnaire variables. Results were expressed as mean ± standard deviation (SD) or percentage. Bivariate analysis used the chi-square or Fischer exact test to assess association between categorical variables. Student t-test or ANOVA, or non-parametric tests were used for quantitative variables. For comparison between 2000 and 2010, common data from KBP-2010-CPHG and KBP-2000-CPHG were compiled and analysed concomitantly (8). Survival curves for SCLC patients in 2010 were estimated according to TNM stage using the Kaplan-Meier method and corresponding 1-year survival estimates with 95% confidence interval (CI) were calculated. Cox proportional hazard model was used to estimate unadjusted or adjusted hazards ratios (HRs) and their 95% CI. Survival time was calculated from the date of diagnosis to the date of death or last visit for alive patients. P values <0.05 were considered as statistically significant.
Results
In 2000 and 2010, respectively, 5,667 and 7,051 patients were included in 137 and 104 centres distributed across France as a whole (including the overseas départements and territories); 80 centres participated in both studies. In 2000 and 2010, respectively, 948 (16.7%) and 968 patients (13.7%) had a SCLC (P<0.001).
Typical patients with SCLC diagnosed in 2010 were heavy active or former male smoker seniors who frequently reported recent weight loss. The disease was commonly diagnosed at advanced stage (Table 1).
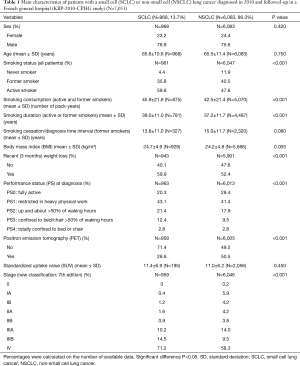
Full table
Compared to NSCLC patients (Table 1), SCLC patients had higher body mass index at diagnosis (P=0.005), but more commonly reported recent weight loss (P<0.001). They had a poorer PS (P<0.001). They were more frequently active smokers (P<0.001) and heavy smokers (P<0.001). Smoking duration was shorter in NSCLC than SCLC patients (P<0.001). SCLC was more frequently diagnosed at advanced stage than NSCLC (P<0.001). In 2010, 35.8% of SCLC patients vs. 44.8% of NSCLC patients (P<0.001) were alive 1 year after the diagnosis.
Compared to 2000 (Table 2), SCLC patients were older (+1.4 years; P=0.008) and more frequently women (P<0.001); they had a better PS at diagnosis (P<0.001). In former-smokers, time interval between diagnosis and smoking cessation increased between 2000 and 2010 (P=0.005). In 2000 and 2010, respectively, 35.8% and 33.1% of SCLC patients (P=0.220) were alive 1 year after the diagnosis.
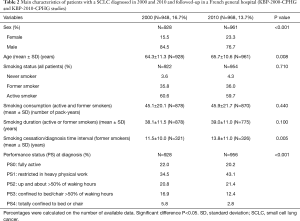
Full table
Table 3 presents the main characteristics of patients with SCLC proven in 2010 according to cancer stage at diagnosis. The percentage of patients with recent weight loss and with a PS >2 increased with tumour stage (P=0.001 and P<0.001, respectively). No other statistically significant difference was observed in patients and tumour characteristics.
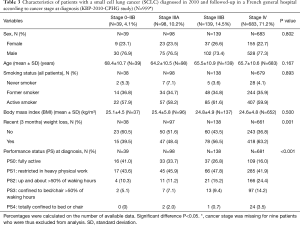
Full table
The greater the stage of the cancer, the lower were the percentages of patients with positron emission tomography (PET) (P<0.001) and with a file discussed during a multidisciplinary team meeting (P=0.031) (Table 4). Regarding initial therapy (Table 4), the greater the stage of the cancer, the lower was the percentage of patients with at least one cancer therapy (although the difference between the four groups was not statistically significant; P=0.098). Overall, 27 patients did not receive any therapy and 74 patients exclusively received supportive care. Curative surgery was rare (n=16) and mainly intended for patients with stage 0–IIB cancer. Palliative irradiation (n=110) was mainly intended for patients with stage IV cancer. Radiochemotherapy was frequent (n=146) and usually concomitant (n=83). Chemotherapy alone was usually palliative (n=684). Platinum-based regimen (cisplatin and carboplatin) and etoposide were the most frequently administered drugs (n=434 and n=390, respectively). Third generation agents (mainly vinorelbine, n=15) were rarely prescribed as initial therapy (n=36) and when prescribed were mainly prescribed in patients with stage IV cancer (n=20). Overall, four patients received one targeted therapy (three patients with stage IV cancer received antiangiogenic therapy combined with non-targeted therapy). No significant difference was observed between patients with stages IIIA and IIIB cancer in the frequency of use of radiochemotherapy, palliative chemotherapy, cisplatin, carboplatin, or other non-targeted therapy such as etoposide (data not shown, P=0.337, P=0.181, P=0.483, P=0.412, and P=0.23, respectively).
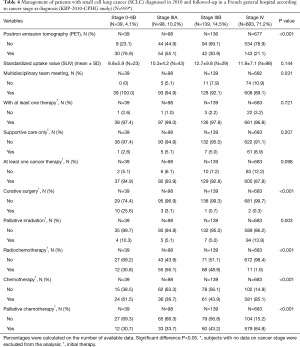
Full table
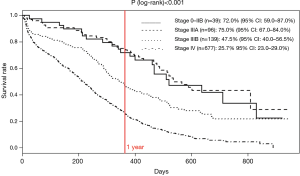
One-year survival rates significantly varied according to cancer stage at diagnosis (P<0.001), ranging from 75% for stage IIA to 25.7% for stage IV (Figure 1). In reference to stage 0–IIB, the HR was 0.92 (95% CI: 0.6–1.5; P=0.760), 1.8 (95% CI: 1.1–2.8; P=0.019) and 3.4 (95% CI: 2.2–5.3; P<0.001) for stage IIIA, IIIB, and IV, respectively. According to whether or not they underwent curative surgery, respectively, 1 and 8 of the 10 and 27 patients with stage IA–IIB cancer and known vital status were died at the end of the follow-up period.
Discussion
The present study confirmed the main characteristics of SCLC patients and showed the impact of societal changes occurred in 10 years on these characteristics. SCLC patients were mainly active or former male smokers whose lung cancer was belatedly diagnosed (10). The percentage of women among SCLC patients has increased in 10 years which reflected the increased proportion of women among smokers in France (9,11). SCLC patients were older and had a better PS at diagnosis in 2010 than in 2000, which possibly reflected increased life expectancy accompanied by increased healthy life expectancy. Moreover, the percentage of SCLC patients followed in the respiratory departments of the French general hospital has decreased in 10 years; however, due to the increased number of new cases of lung cancer (2000: n≈20,000; 2010: n≈37,000) (12), the number of SCLC patients seen each year in each department was stable: approximately ten SCLCs per centre.
One-year survival remained stable from 2000 to 2010 in SCLC patients and poorer than in NSCLC patients. The lack of improvement in 1-year mortality rate in 10 years probably reflected the fact that SCLC remained frequently diagnosed at advanced stage and the lack of improvement in SCLC management. Although cancer stage at diagnosis is a major prognosis factor, we could not compare cancer stages in SCLC between 2000 and 2010 in the present study as the TNM classification has changed. Moreover, the development of the use of new tools such as PET-scan between 2000 and 2010 impacted cancer TNM classification, and consequently therapeutic strategy.
As SCLC is a highly metabolic tumor that avidly takes up fluorodeoxyglucose (FDG), FDG-PET is therefore an attractive modality for SCLC staging and has been used to upgrade patients with extensive disease (10). In a study including 120 SCLC patients, results after FDG-PET were compared with those of conventional staging procedure. Only 1 out of the 120 patients was incorrectly staged by FDG-PET (13). In another study with 18 patients, FDG-PET showed a more extensive disease in 2 of the 3 patients for which FDG-PET and conventional staging disagreed (14). In a third study including 21 patients (39 PET scan examinations), staging was identical when the PET results and the sum of other staging procedures were compared (15). With PET-scan, 9% of SCLCs were “upstaged” and 4% “down staged”. This examination can thus drive therapeutic strategy (radiochemotherapy or surgery).
The present study highlighted the importance in 2010 of the stage of cancer at diagnosis on prognosis. In particular, it showed that 1-year survival was strongly better in patients with stage IIIA as compared with stage IIIB (75% vs. 47.5%). As no obvious difference in the management of stage IIIA and stage IIIB SCLC emerged during the study, it can be hypothesized that difference in prognosis was mainly due to T4 + N2 and N3 cancers among stage IIIB SCLCs and their increased radiation field.
The Veterans Administration Lung Study Group (VALSG) classification of lung cancer remains the most frequently used classification. It classifies patients into two groups: limited (LD) and extensive (ED) disease. The LD group includes patients with primary tumor and nodal involvement limited to one hemithorax, whereas ED group includes all other patients. Approximately, 30% of SCLC patients had LD at diagnosis; their median survival was 18–23 months (vs. 8–10 months in ED patients) and 20% will be long survivors. LD patients can benefit from curative irradiation. In 1987, the International Association for the Study of Lung Cancer (IASLC) published a consensus report in accordance with the TNM. According to the IASLC, the LD group includes all patients without distant metastasis (i.e., stages I to III) and the ED group all patients with stage IV disease (10). The IASLC staging criteria were expected to better predict prognosis according to cancer stage (16). Some studies have also highlighted the difference in prognosis within LD patients taking into account mediastinal lymphadenopathy (17). In a study performed in 1990, the authors concluded that it was necessary to optimize SCLC classification (18). In 2007, the IASLC concluded that TNM was essential to differentiate stages I, II and III whose prognosis was clearly different (19). Survival of 7,995 LD patients were retrospectively analyzed by Patel et al. (20). Among these patients, 45% had a stage IIIB disease (quasi-exclusively due to T4 tumor) and 24% a stage IIIA disease (usually due to N2 disease). In this study, both T and N parameters were significant independent risk factors of overall survival (P<0.001). These data and our results suggest that diseases of stage IIIA and stage IIIB have to be managed differently.
Before the 1970s, surgical resection was used for the management of LD but this therapy has been then supplanted by irradiation based on the data from the British Medical Research Council, which demonstrated that irradiation led to better overall survival in LD (10). Surgical resection which has been showed to improve overall survival in some studies (21) but fails to improve survival in the study by Lad et al. (22) which included 340 patients with stages I to IIIB SCLC. However, some retrospective studies showed an 86% benefit of surgery for stages I and II on 5-year survival (23), a 47% benefit of surgery for stages IA to IIB (24,25), and decreased overall survival with increasing stages. Results of this study also showed that surgery improved overall survival for N0, N1, and N2 (N3 stages were excluded from the study) and postoperative radiotherapy for N2 subgroup but not for N0 and N1 subgroups. Management of N2 SCLC will therefore depend of irradiation. In the beginning, chest irradiation in SCLC was broad and covered the entire mediastinum. Currently, with further technical progresses, radiation field is limited and toxicity decreased (26). A phase III study performed in 471 patients with limited SCLC (27) showed that 5-year survival increased with radiotherapy given twice-daily (1.5 Gy, 30 fractions) compared with once-daily (1.8 Gy daily in 25 fractions) (P=0.04). Moreover, several studies report that survival was improved when radiotherapy was given within the 30 days following chemotherapy (P=0.0003) (28). Currently it seems that (I) therapeutic strategy is rarely surgery followed by chemotherapy for T0-2 N0-1 SCLC; (II) surgery is to be discussed on a case-by-case basis after adjuvant chemo-radiotherapy for N2 SCLC; (III) therapeutic strategy is selective irradiation of lymph nodes in cases of mediastinal adenopathy (29,30). Irradiation is also proposed in particular in cases of lymphadenopathies that have decreased under treatment or in cases of adjacent or supraclavicular lymphadenopathy (31). Shepherd et al. (19) who studied the impact of TNM classification on SCLC management, confirms that all limited SCLC cannot today have the same treatment, and that patients with different prognoses must be identified using TNM classification. No previous studies have investigated a difference between N2 and N3 or T3 and T4 SCLCs.
SCLC management requires the most efficient staging of mediastinal involvement to be sure to not disregard a surgical stage and to identify N2 SCLC. As compared with its previous version, the new version of the TNM classification is expected to better identify prognostic factors and better guide therapeutic strategy in terms of irradiation. PET would be in close future one of the most efficient tool to optimize staging. This examination and possibly other methods such as endobronchial ultrasound (EBUS) would allow N2–3 and T3–4 stages to be differentiated. Radiation techniques including bold techniques (chemotherapy and then radiochemotherapy with stereotactic, adjuvant…) could be performed.
The results of our study confirm those of other studies that highlight the presence of distinct groups within the localized and N2 and N3 SCLCs, and the widespread use of PET-scan. It seems appropriate to propose a different strategy between N2 and N3 SCLCs. N3 SCLCs reflect the rapid evolution of the disease and will benefit from a classic radiochemotherapy. N2 SCLCs could benefit in the future from different multimodal type including stereotactic irradiation and chemotherapy. The place of surgery in case of objective response after chemotherapy would have to be discussed on a case-by-case basis. A study on the management of stage IIIA SCLC would be helpful to confirm these data.
The results of our study also confirm that current guidelines are usually being followed (31). However, there were some discrepancies between the guidelines and our results: although radiochemotherapy is recommended in LD, in our study, only 43.9% and 51.1% of stage IIIA and IIIB patients underwent radiochemotherapy. However, this finding must be interpreted with caution as patients could have been initially treated by chemotherapy and radiotherapy organized in a second time.
Finally, the study, whose strengths and limits have been previously discussed (8,32), confirms the poor 1-year survival in SCLC. It also confirms the impact of the cancer stage at diagnosis on survival rate and, in particular, the discrepancy between 1-year survival and management for stage IIIA and stage IIIB SCLCs, demonstrating the interest of the reintroduction of the TNM classification and the use of new diagnostic techniques, such as PET-scan, to offer more appropriate strategy for each patient.
Acknowledgments
The authors would like to thank all the members of the steering committee and all the chest physicians who have actively participated in this study (see list hereafter). They also thank Mrs Laurent and Le Gall for statistical analysis, and Mrs Petit (Margaux Orange) and Péretz for their help in preparing this article. KBP-2010-CPHG study was promoted by the Collège des Pneumologues des Hôpitaux Généraux (CPHG) with the help of the endowment fund Recherche en Santé Respiratoire of the CNMR, Pneumologie Développement, and funded by the following laboratories: AstraZeneca, BMS, Boehringer Ingelheim, Chugai, GlaxoSmithKline, Lilly France, MSD, Novartis, Pierre Fabre Oncologie, Pfizer, Pneumologie Développement, Roche, and Sanofi-Aventis.
Steering committee: Michel Grivaux, Chrystèle Locher, Didier Debieuvre, Bernard Asselain, François Blanchon, Daniel Coëtmeur, Thierry Collon, Charles Dayen, François Goupil, Francis Martin, Olivier Molinier, Jacques Le Treut.
Investigators: Dr Clarot, Leleu, Abbeville; Dr Le Treut, Aix-en-Provence; Dr Borrel, Albi; Dr Lafourcade, Martin, Angoulême-Saint-Michel; Dr Hominal, Annecy; Dr Mouroux-Rotomondo, Antibes-Juan-Les-Pins; Dr Dubos-Arvis, Argentan; Dr De Cremoux, Argenteuil; Dr Lierman, Arras; Dr Gay, Aubagne; Dr Dion, Aubenas; Dr Virally, Aulnay-sous-Bois; Drs Barbieux, Hakim, Lemonnier, Auxerre; Dr Tagu, Bar-le-Duc; Dr Mouries, Bastia-Furiani; Drs Mathieu, Nocent-Ejnaini, Bayonne; Dr Debieuvre, Belfort-Montbéliard ; Dr Portel, Bergerac; Dr Goutorbe, Béziers; Drs Beynel, Braud, Perrichon, Bourg-en-Bresse; Drs Adam, Allain, Khayat, Lévy, Bourges; Drs Gentil le Pecq, Ravel, Bourgoin-Jallieu; Dr Remignon, Briey; Dr Le Tinier, Briis-sous-Forges-Bligny; Drs Barre, Farny, Cahors; Drs Duval, Perrin, Cannes; Dr Berthiot, Chalons-en-Champagne; Drs Frappat, Kelkel, Chambéry; Dr Le Poulain-Doubliez, Charleville-Mézières; Dr Lamotte, Chateauroux; Dr Simon, Chaumont; Dr Dumont, Chauny; Dr De Luca, Chevilly-Larue; Dr Masson, Cholet; Drs Hammerer, Levy, Meyer, Moreau, Oster, Colmar; Drs Belle, Dehette, Loutski, Compiègne; Drs Belmekki, Ménager, Salmon, Corbeil-Essonnes; Dr Crequit, Le Lann, Creil; Dr Bernier, Dinan; Drs Maëtz, Tavernier, Douai; Dr Barrière, Draguignan; Dr Martin, Dreux; Dr Deniel, Eaubonne; Dr Hauss, Elbeuf-Louviers-Val-de-Reuil; Dr Carbonnelle, Epernay; Drs Collignon, Pontier, Epinal; Dr Mahmoud, Evreux; Dr Merzoug, Fougères; Drs Boudoumi, Desurmont-Salasc, Fréjus-Saint-Raphaël; Dr Thomas, Gap; Dr Tizon-Couetil, Granville-Avranches; Dr Figueredo, Grasse; Dr Badour, Guingamp; Dr Fournier, Hénin-Beaumont; Dr Bedossa, Lagny-sur-Marne; Dr Lemerre, La Rochelle; Dr Berruchon, La-Roche-sur-Yon; Dr Dujon, Le-Chesnay-Versailles; Drs Raffy, Zaegel, Le-Coudray-Chartres; Dr Peureux, Le-Havre; Drs Goupil, Molinier, Le-Mans; Dr Ciobanu, Lens; Dr Florea, Lens; Dr Marcos, Libourne; Dr Oliviero, Longjumeau; Dr Perrichon, Lons-Le-Saunier; Dr Vabre, Lourdes; Drs Dot, Peloni, Lyon-Desgenettes; Drs Blanchet-Legens, Vuillermoz-Blas, Lyon-St-Joseph-St-Luc; Dr Larive, Mâcon; Dr Auliac, Mantes-la-Jolie; Dr Belkaïd, Martigues; Drs Grivaux, Locher, Meaux; Dr Di Mercurio, Melun; Dr Paillot, Metz; Dr Leroy-Terquem, Meulan-les-Mureaux; Dr Benaicha, Montargis; Dr Duvert, Montélimar; Drs Collon, Piquet, Montfermeil-Le-Raincy; Dr Rangasamy, Mont-Saint-Martin; Dr Renault, Morlaix; Drs Belhadj, Marcuccilli, Moulins; Drs Bombaron, Debieuvre, Neidhardt, Mulhouse; Dr Saillour, Nanterre; Drs De Faverges, Herman, Nevers; Drs Bourlaud, D’Arlhac, Niort; Dr Fesq, Nouméa-Nouvelle-Calédonie; Dr De Groote, Oloron-Sainte-Marie; Drs Dixmier, Lemaire, Orléans; Dr Perrus, Paimpol; Dr Ferrer-Lopez, Papeete-Tahiti; Dr Genety, Paray-le-Monial; Dr Renault, Pau; Dr Lacroix, Périgueux; Dr Choma, Perpignan; Dr Fraboulet, Pontoise; Dr Galloux, Quimper; Dr Julien, Rodez; Drs Bautin, Bolard, Brichet-Martin, Cavestri, Just, Lelong, Salez, Steenhouwer, Roubaix; Drs Coëtmeur, Leveiller, Saint-Brieuc; Drs Benothman, Jouveshomme, Saint-Germain-en-Laye-Poissy; Dr Goarant, Saint-Malo; Drs Marty, Sandron, Saint-Nazaire; Drs Huchot, Paganin, Saint-Pierre-Réunion; Drs Boutemy, Dayen, Lecuyer, Saint-Quentin; Dr Kasseyet-Kalume, Salon-de-Provence; Dr Brolly, Saverne; Dr Jeandeau, Sainte-Feyre; Dr Kassem, Sedan; Dr Legrand-Hougnon, Soissons; Dr Botrus, Thionville; Dr Romand, Thonon-Les-Bains; Dr Delclaux, Troyes; Dr Brun, Riou, Valence; Drs Debieuvre, Gury, Vesoul ; Drs Boyer, Marichy, Vienne; Dr Falchero, Villefranche-sur-Saône; Dr Cuguilliere, Villenave-d’Ornon; Dr Razafindramboa, Villeneuve sur lot; Dr Fouret, Villeneuve-Saint-Georges.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocols were approved by French Information Technology and Freedoms Commission (CNIL) on 02 August, 2000 (No. 900019) and 11 January, 2010 (No. 909479). The KBP-2010-CPHG protocol was also approved by the advisory committee on research information processing in the health field (CCTIRS) on 19 November, 2009. The ethics committee of the French Society of Pneumology confirmed the observational nature of the study on 23 April 2010 (No. 2010–008). All patients were duly informed of the study objectives and requirements and gave oral consent before inclusion.
References
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Inoue M, Sawabata N, Okumura M. Surgical intervention for small-cell lung cancer: what is the surgical role? Gen Thorac Cardiovasc Surg 2012;60:401-5. [Crossref] [PubMed]
- National cancer Institute. Surveillance, Epidemiology, and End Results program (SEER). Available online: http://seer.cancer.gov/csr/1975_2012/browse_csr.php
- Haddadin S, Perry MC. History of small-cell lung cancer. Clin Lung Cancer 2011;12:87-93. [Crossref] [PubMed]
- Blanchon F, Grivaux M, Collon T, et al. Epidemiologic of primary bronchial carcinoma management in the general French hospital centers. Rev Mal Respir 2002;19:727-34. [PubMed]
- Blanchon F, Grivaux M, Asselain B, et al. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol 2006;7:829-36. [Crossref] [PubMed]
- Grivaux M, Locher C, Bombaron P, et al. Study KBP-2010-CPHG: inclusion of new cases of primary lung cancer diagnosed in general hospital pneumology departments between 1st January and 31 December 2010. Rev Pneumol Clin 2010;66:375-82. [Crossref] [PubMed]
- Locher C, Debieuvre D, Coëtmeur D, et al. Major changes in lung cancer over the last ten years in France: the KBP-CPHG studies. Lung Cancer 2013;81:32-8. [Crossref] [PubMed]
- Debieuvre D, Locher C, Neidhardt AC, et al. Ten-year evolution in non-small-cell lung cancer according to sex. Results of the KBP-2010-CPHG study by the College of General Hospital Respiratory Physicians. Rev Mal Respir 2014;31:805-16. [Crossref] [PubMed]
- Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology 2008;22:1486-92. [PubMed]
- Levi F, Bosetti C, Fernandez E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer 2007;121:462-5. [Crossref] [PubMed]
- INCa. The cancer situation in France in 2010. Collections rapports & syntheses. Boulogne-Billancourt; November 2010.
- Brink I, Schumacher T, Mix M, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614-20. [Crossref] [PubMed]
- Chin R Jr, McCain TW, Miller AA, et al. Whole body FDG-PET for the evaluation and staging of small cell lung cancer: a preliminary study. Lung Cancer 2002;37:1-6. [Crossref] [PubMed]
- Kut V, Spies W, Spies S, et al. Staging and monitoring of small cell lung cancer using [18F]fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET). Am J Clin Oncol 2007;30:45-50. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM Stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Shepherd FA, Ginsberg RJ, Haddad R, et al. Importance of clinical staging in limited small-cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol 1993;11:1592-7. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol 1990;8:1563-74. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Patel S, Macdonald OK, Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer 2009;115:842-50. [Crossref] [PubMed]
- Jones CD, Cummings IG, Shipolini AR, et al. Does surgery improve prognosis in patients with small-cell lung carcinoma? Interact Cardiovasc Thorac Surg 2013;16:375-80. [Crossref] [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-323S. [Crossref] [PubMed]
- Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. [Crossref] [PubMed]
- Bischof M, Debus J, Herfarth K, et al. Surgery and chemotherapy for small cell lung cancer in stages I-II with or without radiotherapy. Strahlenther Onkol 2007;183:679-84. [Crossref] [PubMed]
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. Oncologist 2010;15:187-95. [Crossref] [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [Crossref] [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol 2006;24:1057-63. [Crossref] [PubMed]
- van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2010;77:329-36. [Crossref] [PubMed]
- Shirvani SM, Komaki R, Heymach JV, et al. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;82:e91-7. [Crossref] [PubMed]
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Debieuvre D, Oster JP, Riou R, et al. The new face of non-small-cell lung cancer in men: results of two French prospective epidemiological studies conducted 10 years apart. Lung cancer 2016;91:1-6. [Crossref] [PubMed]


