Effect of telling patients their “spirometric-lung-age” on smoking cessation in Japanese smokers
Introduction
Cigarette smoking remains a significant public health problem internationally, despite some modulation by tobacco control efforts in several countries. However, smoking rates have not yet declined sufficiently (1). In our country, the smoking rate in adults was 19.3% in 2014 (male 32.2%, female 8.2%) (2). The trend indicated a slow but consistent decline since 1997, but the smoking rate remains almost unchanged during the past 4 years (2). Tobacco control efforts, which comprise media campaigns, increased taxes on cigarettes, restricted smoking areas, plain packaging, and smoking cessation programs, etc., have been conducted by governments and society. For smoking cessation, several modalities of evidence-based treatments for tobacco-dependence (brief advice from health professionals, toll-free quit lines, pharmacotherapy such as nicotine replacement therapy and varenicline, etc.) are available in many countries (3). Additionally, numerous efforts are directed at increasing smoking quit rates. For example, one report described several motivational interventions to encourage smoking cessation that successfully raised the quit rate (4). Nurse-interviewing and telephone- or web-based programs have been demonstrated to efficiently raise subjects’ efforts to stop smoking (5-7). However, the overall effects of these programs have often been inconsistent and the prevalence of smoking has not reduced sufficiently, even with these various efforts.
Spirometry can detect patients at a high risk of developing chronic obstructive pulmonary disease (COPD) among smokers in general practice. This lung function test leads to an early detection of airflow obstruction [forced expiratory volume in 1 second (FEV1) <80% of predicted], which appears to develop approximately in a quarter of smokers with chronic cough (8). The early detection of ventilatory impairment in individuals who are at risk of developing COPD and education of patients about smoking-related lung damage is a positive means of influencing smokers to engage in cessation programs. Furthermore, if even subtle abnormalities in pulmonary function are proven by spirometry, that result may provide an educational opportunity for asymptomatic smokers. Unfortunately, though, the detection of abnormalities in pulmonary function has so far failed to raise smoking quit rates (9). One of the possible causes seems to be that smokers are poorly educated in this subject, suggesting that familiar or understandable terms rather than scores showing defective pulmonary function may be required to motivate smokers to quit smoking.
The concept of “spirometric-lung-age (SLA)” (the age of an average person who has, for example, FEV1 equal to a subject) was developed in 1985 as a way of making spirometry data easier to understand (10) and also as a potential psychological tool to show smokers the apparent ageing of their lungs (11). However, the effect of these motivational interventions may be influenced by multiple factors including the differences in racial backgrounds and cultural practices (12), smoking cessation programs, and so on. Thus, we evaluated the effect of telling patients their SLA on the smoking quit rate among Japanese smokers at our outpatient clinic.
Methods
The standardized smoking cessation program in Japan
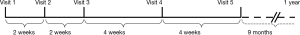
As shown in Figure 1, we performed this treatment according to the standardized program for smoking cessation approved by the Ministry of Health, Labour and Welfare in Japan. This program consists of five visits (Visits 1 to 5) during 12 weeks for treatment with either a nicotine patch or varenicline as medicinal support for smoking cessation. The medical costs are covered by our health insurance system if the treatment adheres to this standardized program. However, no further treatments for smoking cessation are covered by this health insurance system for 1 year after Visit 1, irrespective of whether patients successfully quitted smoking or not. This means that those who quitted successfully have no contact support under the coverage of health insurance system after Visit 5. Additionally, smokers who failed to quit in the program have to wait for 1 year before re-entering the smoking cessation program covered by health insurance.
Intervention by telling SLA
On Visit 1 of the smoking cessation program, all participants were informed of smoking-related health problems and randomly assigned either to the SLA group or control group by odd-even allocation. That is, the participants who consulted the clinic on odd-numbered months were assigned to the control group, whereas those who visited on even-numbered months were placed in the SLA group. The SLA group received education about the natural history of smoking-related airflow obstruction (13) and the concept of SLA (14) before seeing the doctors, whereas the control group did not have that information. The SLA group underwent a standard measurement of pulmonary function [FEV1, forced vital capacity (FVC), FEV1/FVC] with a spirometer, Hi-Checker® (Takara Tsusho Co., LTD, Tokyo, Japan) and estimation of their SLA. SLA was calculated as follows: [0.036× height (cm) –1.178– FEV1 (L)]/0.028 for males and [0.022× height (cm) –0.005– FEV1 (L)]/0.022 for females according to a formula approved by the Clinical Pulmonary Functions Committee of the Japanese Respiratory Society. Accordingly, participants in the SLA group noticed the difference between their personal lung age and chronological age immediately after spirometry. If the SLA was more than the individual’s chronological age, the participants were informed that smoking had damaged their lungs. An explanation followed that smoking cessation would slow down the rate of deterioration of their lung function back to a physiological age-related decline but that the smoking-related lung damage would not be repaired. On the other hand, if the SLA was equal to or less than the individual’s chronological age, the participants were informed that their lungs currently seemed to be normal, but their risk of having other smoking-related health problems would remain. Participants in the control group were informed only that they would receive the standard treatment for smoking cessation, but they were not given any motivational intervention.
On Visit 1, all participants completed Prochaska’s questionnaire about stages and processes of self-change from habitual smoking (15), which surveyed their intention to stop smoking to be classified from the “pre-contemplative” “contemplative” “preparation” to “action” phases. All participants were also given a questionnaire from the Tobacco Dependence Screener (TDS) (16) and were examined for their exhaled carbon monoxide (e-CO) levels (non-smoker, e-CO <3 ppm; Smorkelyzer®, Harada Corp., Osaka, Japan). Both Prochaska’s questionnaire and TDS were obtained by participants’ self-rating. TDS is a standard questionnaire for participants who intend to stop smoking cigarettes in Japan (16-19). Either the nicotine patch or varenicline was prescribed as medication to support smoking cessation according to patients’ preferences. All participants were carefully observed whether they used the medication properly and had adverse events from the medication on every visit to outpatient clinic. We also educated them about benefits from smoking cessation, encouraged to stop smoking or stay abstinent, and then tried to keep them motivated to stop smoking.
Outcome measures
The primary outcome is to establish the smoking quit rate on Visit 5 of the program. Termination of smoking was confirmed by measuring e-CO according to the standardized program. The secondary outcome is whether participants continue abstinence or not. Abstinence or recidivism was confirmed 1 year later by mailing questionnaires.
Ethical considerations
The present study was conducted prospectively and single-blindly at our outpatient clinic from December 2010 to September 2011. The study protocol was reviewed and approved by the institutional ethics committees of Juntendo University Hospital on December 12th, 2010 (approval number: 22-235). Written informed consent was obtained from all participants before inclusion in the study. The researchers will ensure that the study is conducted in compliance with principles of the Declaration of Helsinki and the International Conference on Harmonization guidelines for good clinical practice and applicable legislation.
Statistical analysis
Groups were compared with the unpaired t-test for continuous variables and by Fisher’s exact test or Chi-square test for categorical variables. Multivariate logistic regression analysis was performed to identify factor that significantly associates with smoking quit rate on Visit 5 as well as the rate the quitters on Visit 5 remained abstinence 1 year later. Variables were selected on Visit 5 with a forward stepwise procedure. Variables of age (10-year regarded as 1 unit), gender, group (SLA/control), smoking (100-pack-year regarded as 1 unit), TDS, treatment (varenicline/nicotine patch) and comorbidities (respiratory, cardiovascular, mental, others) were included in the model. For the analysis of 1 year later abstinence, the same variables as on Visit 5 were used in the model with 57 patients who quitted smoking on Visit 5.
For all statistical analyses we used SPSS version 19.0. Data are presented as means ± standard deviation (SD). Differences with P values <0.05 were considered statistically significant.
Results
Baseline characteristics
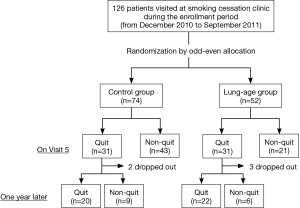
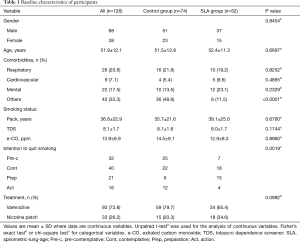
All subjects who visited at our smoking cessation clinic were asked to participate in this study. They decided to visit at our clinic by themselves, were referred to by general practitioners, or introduced to by other clinics in our university hospital. From December 2010 to September 2011, we prospectively recruited 126 subjects and all gave consent to participate in this study (Figure 2); they were assigned to either the control group (n=74) or the SLA group (n=52) by odd-even allocation on the month they visited at our clinic. A total of five participants in both groups were lost from the survey 1 year later since we could not contact them. The baseline characteristics of all participants appear in Table 1. Mean age at participation as an entire group was 51.9±12.1 years old, and 69.8% of participants were male. There was no significant difference in gender, age, smoking status, and pharmacotherapy between control and SLA groups. However, the differences were noted in comorbidities and intention to quit smoking: less frequency of comorbidities classified as others including dyslipidemia, diabetes mellitus, collagen vascular diseases and others, and higher intention to quit smoking in the SLA group.
Full table
Outcomes
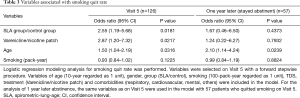
The smoking quit rate on Visit 5 was similar between SLA and control groups (59.6% vs. 41.9%; P=0.0700) (Table 2). However, the proportion of patients who remained abstinent 1 year later became similar in both groups (78.6% vs. 69.0%; P=0.5497) (Table 2). Since the baseline characteristics of SLA and control groups showed the difference in some aspects, we performed multivariate logistic regression analysis to adjust the baseline differences and then to identify factors that significantly associated with smoking quit rates on Visit 5 and 1 year later (Table 3). We found that telling patients their SLA, the use of varenicline, and age were significantly associated with smoking quit rate on Visit 5. On the other hand, age was the factor only associating with stayed abstinent 1 year later.

Full table
Full table
We next analyzed whether there are differences in the clinical characteristics between quit and non-quit in the lung-age group. No significant difference distinguished those who did stop smoking cigarettes from those who did not in SLA, difference between SLA and chronological age, FEV1, FEV1/FVC, or treatment status (Table 4). Multivariate logistic regression analysis failed to identify a statistical difference between quit and non-quit in SLA group (data not shown).
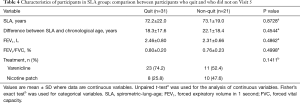
Full table
Discussion
The present study examined the effect of telling patients their SLA on smoking quit rate among smokers at our outpatient clinic. Our study demonstrated that three variables, telling patients their SLA, the use of varenicline, and age, were identified to be significantly associated with smoking quit rate on Visit 5, but only age remained significant for sustained abstinence 1 year later. These results suggest that the motivational intervention of telling patients their SLA is effective and helpful for smokers to quit smoking for the short-term. The exact mechanism by which this intervention achieved its effect remains undetermined. Although the results of spirometry would provide an ideal educational opportunity for asymptomatic smokers to perceive the status of their lung health, that process failed to promote longer term smoking cessation (9), possibly because the lay public generally fails to understand the full meaning of spirometry in the context of long-term health and life span. In contrast, the term “SLA” estimated from spirometry data appears to be easier and more acceptable for smokers. Conceivably, the concept of SLA may effectively draw smokers’ attention to their risk of developing lung diseases. Helping smokers to realize the current status of their lung health would, then, have prompted smoking cessation.
Parkes et al. (11) previously reported the efficacy of telling patients their SLA on smoking quit rates among 561 smokers for 12 months. That study demonstrated that the smoking quit rate increased to 13.6% after telling patients their SLA vs. 6.4% in the control group. However, there is a striking difference in the baseline quit rate (control group, i.e., without motivational intervention by telling SLA) between our outcome and that of Parkes et al. (11). In our program, the quit rate of the control group was 41.9% on Visit 5 and two-third of them stayed abstinent 1 year later. Although the number of participants was quite different between the two studies [126 in ours vs. 561 in Parkes et al. (11)], the results suggest the following interpretations. First, telling patients their SLA appears to induce smokers to quit smoking even when they differ in ethnicity, although ethnic disparities in daily cigarette smoking rates, nicotine dependence, cessation motivation, and knowledge of cessation methods and products have been reported (12). Rather, these disparities may influence the baseline quit rate as seen in the differing outcomes between the report of Parkes et al. (11) and the present study. Secondly, motivational intervention by telling SLA appears to be effective in raising the smoking quit rate irrespective of the program’s length [1 year for Parkes et al. (11) vs. 12 weeks for the present study]. Further study is needed to test whether telling patients their SLA universally promotes smoking cessation regardless of ethnicity and cultural background.
In the present study, the effect of telling SLA on smoking quit rate was lost, considering the relapse rate at 1 year after successful quit on Visit 5: 21.4% (6/28) in the SLA group vs. 31.0% (9/29) in controls. Agboola et al. demonstrated a 55% relapse after abstinence from smoking at week 52 among varenicline users (20), whereas Ebbert et al. showed that abstinence rates from smoking among varenicline users were 32% during 15 through 24 weeks and 27% during 21 through 52 weeks (21). Compared to those reports, the relapse rates seemed to be lower in our study. Factors related to relapse after abstinence from smoking have been attributed to increased alcohol intake and lower numbers of support contacts (22). Unfortunately, we did not survey alcohol intake in our study population. Furthermore, any support contacts were not offered after Visit 5, because health insurance does not cover longer terms under the current smoking cessation program in Japan. Cochrane’s review (4) reported that psychosocial interventions for smoking cessation in patients with coronary heart diseases are effective in promoting abstinence up to 1 year, provided the interventions are of sufficient duration. Accordingly, the great risk of relapse appears to reside inherently in our government-approved program, which the result of our study illustrated. Interestingly, multivariate analysis demonstrated that age was significantly associated with not only the smoking quite rate on Visit 5 but also the rate of remaining abstinent 1 year later. Odds ratio were even higher for the rate of remaining abstinent 1 year later than for the smoking quit rate on Visit 5. The reason for this remains undetermined, but further study on socioeconomic factors may help resolve this issue.
We found the favorable influence of pharmacotherapy from varenicline on the smoking quit rate at Visit 5, but not on the rate of remaining abstinent 1 year later. Several studies demonstrated that both the nicotine patch and varenicline effectively encouraged smoking cessation; however, a higher quit rate was reported for varenicline users (23,24). Kotz et al. described results from their prospective study in which varenicline users had a 3.8 times higher quit rate than subjects who chose the nicotine patch (25). In addition, the relapse rate after abstinence from smoking was demonstrably smaller in varenicline users than nicotine patch users (26). Since this information is widely distributed, this knowledge about pharmacotherapy seems to be the reason why more participants chose varenicline than a nicotine patch in the present study. Despite of different ethnicities, cultural backgrounds, and health-care systems, the influence on pharmacotherapy-related smoking quit rates from varenicline may be consistent.
Some limitations should be addressed in the present study. First, the number of participants in this study was limited as compared with the preceding studies. Secondly, the disparity in the number of participants between control and SLA groups existed due to our method of randomization. Third, individual situations of participants who failed to quit smoking 1 year later were not investigated. Those statistics might have influenced the interpretation of results in our study. Further research should be addressed the comparative effectiveness in the long term.
Conclusions
In conclusion, motivational intervention by familiar or understandable terms, telling patients their SLA, may be a noticeable way to smoking cessation, leading to reduction in the effect of smoking on international public health problem. To make the most of this short-term effect of telling patients their SLA on convincing them to stop smoking, exploring interventions to promote permanent abstinence, such as, periodic support contacts covered by health insurance, is a worthwhile endeavor.
Acknowledgements
We thank Ms. Phyllis Minick for her excellent proofreading of English writing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was reviewed and approved by the institutional ethics committees of Juntendo University Hospital on December 12th, 2010 (approval number: 22-235). Written informed consent was obtained from all participants before inclusion in the study.
References
- Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA 2014;311:164-71. [Crossref] [PubMed]
- Japan Health Promotion and Fitness Foundation [Internet]. Japan. Available online: http://www.health-net.or.jp/tobacco/product/pd100000.html
- Fiore MC, Baker TB. Clinical practice. Treating smokers in the health care setting. N Engl J Med 2011;365:1222-31. [Crossref] [PubMed]
- Barth J, Jacob T, Daha I, et al. Psychosocial interventions for smoking cessation in patients with coronary heart disease. Cochrane Database Syst Rev 2015;7:CD006886. [PubMed]
- Bredie SJ, Fouwels AJ, Wollersheim H, et al. Effectiveness of Nurse Based Motivational Interviewing for smoking cessation in high risk cardiovascular outpatients: a randomized trial. Eur J Cardiovasc Nurs 2011;10:174-9. [Crossref] [PubMed]
- Terry PE, Seaverson EL, Staufacker MJ, et al. The effectiveness of a telephone-based tobacco cessation program offered as part of a worksite health promotion program. Popul Health Manag 2011;14:117-25. [Crossref] [PubMed]
- Mason D, Gilbert H, Sutton S. Effectiveness of web-based tailored smoking cessation advice reports (iQuit): a randomized trial. Addiction 2012;107:2183-90. [Crossref] [PubMed]
- Van Schayck CP, Loozen JM, Wagena E, et al. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002;324:1370. [Crossref] [PubMed]
- Bize R, Burnand B, Mueller Y, et al. Effectiveness of biomedical risk assessment as an aid for smoking cessation: a systematic review. Tob Control 2007;16:151-6. [Crossref] [PubMed]
- Toda R, Hoshino T, Kawayama T, et al. Validation of “Lung Age” measured by spirometry and handy electronic FEV1/FEV6 Meter in pulmonary diseases. Intern Med 2009;48:513-21. [Crossref] [PubMed]
- Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336:598-600. [Crossref] [PubMed]
- Herzog TA, Pokhrel P. Ethnic differences in smoking rate, nicotine dependence, and cessation-related variables among adult smokers in Hawaii. J Community Health 2012;37:1226-33. [Crossref] [PubMed]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-8. [Crossref] [PubMed]
- Morris JF, Temple W. Spirometric "lung age" estimation for motivating smoking cessation. Prev Med 1985;14:655-62. [Crossref] [PubMed]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5. [Crossref] [PubMed]
- Kawakami N, Takatsuka N, Inada S, et al. Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addict Behav 1999;24:155-66. [Crossref] [PubMed]
- Ministry of Health, Labour and Welfare [Internet]. Japan. Available online: http://www.mhlw.go.jp/topics/tobacco/kin-en-sien/manual/index.html/
- Nakamura M, Oshima A, Fujimoto Y, et al. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther 2007;29:1040-56. [Crossref] [PubMed]
- Uchida K. Smoking cessation treatment, analysis of smoking cessation rate, and the reason of lower smoking cessation rate in women compared with that in men. Nihon Kokyuki Gakkai Zasshi 2007;45:673-8. [PubMed]
- Agboola SA, Coleman T, Mcneill A, et al. Abstinence and relapse among smokers who use varenicline in a quit attempt-a pooled analysis of randomized controlled trials. Addiction 2015;110:1182-93. [Crossref] [PubMed]
- Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313:687-94. [Crossref] [PubMed]
- Koçak ND, Eren A, Bog˘a S, et al. Relapse Rate and Factors Related to Relapse in a 1-Year Follow-Up of Subjects Participating in a Smoking Cessation Program. Respir Care 2015;60:1796-803. [Crossref] [PubMed]
- Hsueh KC, Hsueh SC, Chou MY, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology (Berl) 2014;231:2819-23. [Crossref] [PubMed]
- Kaduri P, Voci S, Zawertailo L, et al. Real-world effectiveness of varenicline versus nicotine replacement therapy in patients with and without psychiatric disorders. J Addict Med 2015;9:169-76. [Crossref] [PubMed]
- Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of varenicline versus nicotine replacement therapy for smoking cessation in the "real world". BMC Public Health 2014;14:1163. [Crossref] [PubMed]
- Zhu SH, Cummins SE, Gamst AC, et al. Quitting smoking before and after varenicline: a population study based on two representative samples of US smokers. Tob Control 2016;25:464-9. [Crossref] [PubMed]