Chinese consensus statement on standard procedure and perioperative management of bronchial thermoplasty
Bronchial asthma (also known as asthma) is a common and chronic respiratory disease affecting over 300 million people globally. There are more than 30 million asthmatic patients in China. Furthermore, 5–18% of Chinese asthmatic patients are clinically categorized as severe asthma. Asthma is characterized by the reversible limitation of airflow. Typical pharmacotherapy for asthma includes corticosteroids, β2-agonists, theophylline, leukotriene modifiers, and anticholinergic agents. To date, the chronic airway damage caused by asthma could not be restored by any pharmacotherapy (1,2).
Bronchial thermoplasty (BT) was approved by the US Food and Drug Administration (FDA) in 2010 for the treatment of severe asthma in patients aged 18 years and older. In China, BT was approved for clinical use in late 2013. BT is currently available in many medical institutions nationwide and satisfying clinical results have been achieved (3-5) with strong safety records (2,6,7).
The effectiveness and safety of BT is related to not only the standardization of the actual procedure but also perioperative management: proper patients’ selection, preoperative assessment, postoperative management and follow-up (2,6,7). In order to standardize the technology to improve therapeutic effects, minimize the occurrence of complications and reduce healthcare expenditures in the process, Chinese experts in asthma and BT reviewed the literature and drew on their own experiences to establish this standard practice guideline (3,4,8,9).
The apparatus and rationale for BT
BT is a non-pharmacologic therapy for severe asthma.
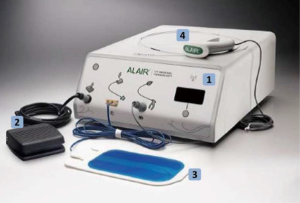
- The BT apparatus: BT is performed clinically using the Alair System (Boston Scientific, Natick, MA, USA), which is comprised of the Alair radiofrequency (RF) controller, a control foot-switch, a negative electrode and Alair Catheter (Figure 1).
- The rationale for BT: the Alair Catheter is inserted via the flexible bronchoscope (ideally with outer diameter no more than 5 mm) with a minimum working channel diameter of 2 mm to the walls of the distal tracheobronchial tree with a diameter of 3 mm minimum. The distal tip of the catheter contains an expandable 4-electrod basket, which is serially deployed in the airway to treat all visible airways sequentially. The RF controller delivers temperature-controlled thermal energy pulses at 65 °C for 10 seconds. This ablation substantially reduces airway hyper-responsiveness, airway obstruction and asthma symptoms through selectively reducing the thicker airway smooth muscles.
Indications and contraindications
Indications
China FDA (CFDA) approved BT for the treatment of severe persistent asthma in patients 18 years and older whose asthma is not well controlled with high dose inhaled corticosteroids and long-acting β-agonists.
As published in the Global Initiative for Asthma (GINA) guidelines, severe asthma is asthma that requires GINA step 4 or 5 treatment for the previous year to prevent it from becoming ‘uncontrolled’ or asthma that remains ‘uncontrolled’ despite the aforementioned treatment.
Uncontrolled asthma is commonly defined as follows:
- Poor symptom control: Asthma Control Questionnaire (ACQ) consistently >1.5, Asthma Control Test (ACT) < 20 (or ‘‘not well controlled’’ according to GINA);
- Frequent severe exacerbations: two or more bursts of systemic corticosteroids (>3 days each) in the previous year;
- Serious exacerbations: at least one hospitalization, ICU stay or mechanical ventilation in the previous year;
- Airflow limitation: after bronchodilator inhalation, persistent airflow limitations still exist (FEV1 <80% predicted, FEV1/FVC <0.7).
Contraindications
Absolute contraindications
- Patients with a pacemaker, internal defibrillator, or other implantable electronic devices;
- Acute myocardial infarction in the last 6 weeks;
- Patients with severe cardiac and pulmonary diseases that make them not suitable for bronchoscopy;
- Sensitivity to anesthetics required to perform bronchoscopy;
- Patients with active bleeding and coagulation dysfunction;
- Patients who have been previously treated with BT.
Relative contraindications
- Patients unable to withhold anti-coagulant and anti-platelet agents;
- Severe damaged lung function by uncontrolled asthma;
- History of near-fatal asthma;
- Other uncontrolled comorbidities.
Pre-procedure assessment
To facilitate a safe procedure, adequate pre-procedure preparation is critical. Pre-procedure preparations should include the followings:
Diagnosis and assessment
Prior to BT treatment, the diagnosis of severe asthma should be confirmed. Lung function test, bronchial provocation and dilation tests, the measuring of airway inflammatory biomarkers (e.g., induced sputum cytology examination), fractional exhaled nitric oxide (FeNO), peak expiratory flow (PEF) and its variability, chest radiological examination [e.g., chest high resolution CT (HRCT)] and the assessment of serum immunity [autoantibody to nuclear antigen (ANA), anti-neutrophil cytoplasmic antibodies (ANCA)] should be accessed. After the diagnosis of asthma is confirmed, non-asthmatic diseases (e.g., bronchiectasis, COPD, bronchiolitis obliterans, and central airway obstructive diseases) should also be ruled out as they may present with asthma-like symptoms such as wheezing, breathlessness and chest tightness.
Assessing the control of environment factors and inhaler technique
Environment factors such as indoor and outdoor allergens, cigarette smoke exposure, atmospheric contamination, occupational exposure should be minimized. “Uncontrolled” asthma due to incorrect inhaler technique should be excluded.
Assessing asthma control and medication adherence
In order to assess the impact on the quality of life from asthma, ACQ should be administered (such as the ACT). Objective measurements of lung function should be performed, including the forced expiratory volume in one second as a percentage of predicted (FEV1%pred), PEF and its variability, airway inflammatory biomarkers (e.g., induced sputum cytology examination) and FeNO, as well as the Mini Asthma Quality of Life Questionnaire (MiniAQLQ). Assessment of patient adherence to medication should be performed.
Assessing patient suitability
- Pulmonary function test: the literature supports the safety of BT in patients with FEV1 greater than 60%pred after bronchodilators treatment. There is published literature and recently presented poster at the American Thoracic Society meeting in Washington, DC 2017 establishing safety in patients with FEV1 below 60%pred, but the literature is not as robust.
- Chest HRCT: HRCT scans mainly reveal lung structure changes, particularly pulmonary emphysema and bronchiectasis. For patients with regional emphysema, segmental or lobar bronchiectasis, BT treatment should be avoided in the affected segments/subsegments.
Assessing comorbidities
Comorbidities play central roles in the risk of symptom fluctuation in severe asthma. The assessment of comorbidities is standard of care for every patient with severe asthma. Common comorbidities include hypertension, diabetes, obesity, obstructive sleep apnea-hypopnea syndrome (OSAHS), rhinitis or sinusitis, gastro-esophageal reflux disease. Each comorbidity should be optimized prior to BT. Appropriate respiratory support should also be given during the recovery period, including albuterol nebulization and positive pressure mask-ventilation if needed.
Before BT treatment, the physician should obtain the patient’s detailed medical history (such as previous asthma exacerbations, hospitalizations, emergency room visits and mechanical ventilations) to evaluate the asthma severity and the risks during and post procedure in order to determine suitable post-procedure care for the patient.
Preoperative assessment of routine bronchoscopy
BT is an interventional treatment for asthma that is performed with a bronchoscope. In order to ensure the safety of routine bronchoscopy, standard evaluations with coagulation tests, hepatitis virus serology and HIV antibody detection should be done. Blood pressure, heart rate, electrocardiogram, arterial blood gas and transcutaneous blood oxygen saturation should be measured before BT treatment. The heart function should also be evaluated via echocardiography or plasma brain natriuretic peptide (BNP) detection if necessary. Medications prohibited by bronchoscopy should be stopped.
Pre-procedure medications and anesthesia management
Preoperative preparation
- Prophylactic use of drugs: prophylactic prednisone or oral corticosteroids (equivalent to 30–50 mg/d of prednisone) should be taken 3 days before the procedure, on the day of the procedure and 1 day after the procedure. An alternative strategy of 2 days before, day of procedure, and 2 days after has been used. The corticosteroids are used to decrease preoperative airway inflammation, enhance asthma stability, and reduce local edema and inflammation of airway wall injury by BT. The published safety studies of BT utilized this steroid regimen.
- Preoperative preparation: patients should be educated on the aims, benefits, risks and the overall operation process of BT. The patients should not eat or drink at least six hours before the procedure if being done with light sedation or minimum eight hours if under general anesthesia.
- Intraoperative monitoring: continuous SpO2, capnography, ECG, blood pressure and airway pressures.
- Premedication: pre-procedure medications should be given 30 minutes before the procedure. (i) Combined albuterol and ipratropium bromide inhalation at a dose of 2.5 mL or nebulized albuterol (4 to 8 puffs); (ii) antisialogogue: anticholinergic agent atropine (0.5 mg im) or racanisodamine hydrochloride (namely 654-2, 10 mg im); (iii) anxiolytic: if the patient experiences anxiety, midazolam (1 to 2 mg); (iv) may be given. Particular attention should be paid if the patient is sensitive to any aforementioned drugs.
Anesthesia
BT may be performed under general anesthesia (total intravenous anesthesia or gas anesthesia), local anesthesia combined with sedation or simply sedation, in accordance to different situations.
- Local anesthesia combined with sedation and analgesia: BT is optimized when performed under moderate sedation. When in this state, patients generally can tolerate the procedure. It is simple with low cost and high safety. However, a small proportion of patients may have difficulty controlling their cough and impeding the performance of the procedure.
- Local anesthesia: local anesthesia should be implemented in accordance to the standards of routine bronchoscopy, such as 2% lidocaine inhalation through the nasal passage. Special attention should be paid to the enhanced anesthetization of the epiglottis, supraglottic and subglottic airways and airways that are to be treated. Repeated dosage should be given if it is necessary (every 30–40 minutes). However, the total dose should be no more than 8.2 mg/kg.
- Sedation: midazolam 1–2 mg is the loading dose. A maintenance dose should be given if needed.
- Analgesia: additional analgesics helps patients obtain better tolerance for the procedure. Sufentanil 5 µg loading dose may be used and a maintenance dose should be given in a certain time interval if needed (sufentanil is recommend abroad, but is not advised in asthmatics patients according to Chinese pharmacopoeia).
- Total intravenous anesthesia: if a patient cannot tolerate the procedure with moderate sedation and topic anesthesia, total intravenous anesthesia should be used. The typical practice is intravenous anesthesia and ventilation via a laryngeal mask, with or without muscle relaxants. An endotracheal tube can be utilized if the clinical parameters require it. With positive pressure ventilation and deep sedation, there is relatively good airway dilatation and a calm, non-coughing patient. The entire process requires anesthesiologist involvement and ventilation techniques support.
- Post-anesthesia care: post-anesthesia care should be carried out according to different post-procedure care guidelines that are based on various anesthetic methods that were used during the case.
Technique
BT is performed over three separate bronchoscopy sessions. Each BT session is done a minimum of 3 weeks apart. By convention, the first procedure is done in the right lower lobe. The left lower lobe is in the second session and both upper lobes (including lingual) are in the final session. The right middle lobe is not treated per protocol.
Performing BT
- Link bronchoscope to Alair System and make sure all devices are fully functional.
- Examine all airways through routine bronchoscopy. Suspend or postpone the procedure (otherwise avoid regional airways) for any of the following reasons: (i) bronchus are unusually edematous or inflamed, which cannot be explained by asthma; (ii) extensive and/or prolonged bronchoconstriction; (iii) airways accessed in previous bronchoscopy session do not seemed to be sufficiently healed; (iv) presence of purulent or abnormally tenacious sputum or mucus plugging; (v) inability to access airways caused by excessive secretions, excessive coughing or tortuous anatomy; (vi) for any other reasons the physician feels that the treatment should be terminated.
- Plan the activation sequence: the activation sequence planning is crucial to the success of treatment. If the bronchus is approached in a random way, some bronchus might be treated twice whereas other bronchus might be skipped entirely. We recommend a systematic approach from distal to proximal bronchus and from small to large angle airways. In general, the activation is performed from distal to proximal, from one segment to immediate adjacent segments across the lobe until all accessible bronchus are carefully identified and treated only once. For example, activation sequence of the right lower lobe could be anterior basal segment followed by the lateral and posterior basal segments. The medial basal segment is then treated, followed by the distal portion of the right lower lobe bronchus up to the level of the dorsal segment. Finally, the dorsal segment is treated. Within each segment, the subsegmental bronchus should also be treated in a systematic manner, from superior bronchus to inferior bronchus or from the right part of bronchus to the left parts.
- Activation. After treatment planning, the bronchoscope is navigated to the most distal region obtainable (usually III–IV class bronchi). The more distal bronchi that can be treated beyond the scope are identified (diameters ¡Ý3 mm, usually V-class bronchi) and the bronchoscope is positioned in the targeted site in clear bronchoscopic view. The catheter is then inserted into the working channel of the bronchoscope and pushed through until the distal end. Once placed at the targeted region, the electrode array is expanded until the four electrode wires firmly contact the airway wall. Be cautious to not over-expand the electrodes, as this may result in distortion of the electrode array. When the electrode array is properly positioned and expanded, the doctor delivers energy by pressing and releasing the controller footswitch. The controller will deliver energy automatically for approximately ten seconds in accordance to preset treatment parameters. After activation, the doctor partially collapses the electrode array and repositions it proximally about 5 mm (5 mm mark on catheter) adjacent to but not overlapping the previous activation sites. This process is repeated along the entire length of each targeted airway and progresses from airway to airway as determined during treatment planning. Anatomic landmarks should be used as references for electrode array position due to the potential for relative motion among the bronchoscope, catheter and airways. The use of a ‘‘map’’ of the airways to plan and track the progression of the treatment for each session is recommended.
- After all accessible airways are treated, all airways should be checked again and secretions should be cleared.
Handling precautions
- Effect of respiratory movement: as the lower lobe bronchus move significantly with breathing, the active catheter needs to be carefully positioned consistently with the bronchial. Allow for upper lobe bronchial breathing when the mobile range is relatively small. Catheter displacement during activation will result in a misfire and inadequate energy being delivered.
- The angle of the catheter bend: if the bronchial angle of the bilateral superior lobe apicoposterior segment and the lower lobe dorsal segment is larger, it is sometimes difficult for the catheter to pass. Its bending angle relative to the bronchoscope is prone to deformation and loss of normal oval structure, resulting in poor contact when the electrode is open. This will negatively impact the treatment effect. The bronchoscopy with 4 mm outer-diameter is recommended. The first step is to activate the small angle bronchi; the last step is to activate larger angle bronchi in order to avoid over-bending the catheter. Since the catheter is expensive, special attention should be paid to prevent being damaged. It is best to have an extra catheter for each operation. If one of the prongs of the electrode becomes bent inward instead of bowing outward like normal, there are two steps to try and fix this. First, simply run your fingers over the closed catheter and pull on the distal tip. This force will usually result in the catheter bowing in the correct direction. If this fails, then partially deploy the electrode and use a toothpick to push on the inward bending electrode to push it to the correct outward position.
- Because of the large number of bronchial grades and long operation time, it is easy to leave bronchi untreated. Therefore, the activation sequence should be in accordance with the planned order. Time tracking by assistants on-site is needed. It must be emphasized that the ablation cannot be repeated because it would damage the airway wall and could result in irreversible damage.
- Intraoperative adjuvant treatment: since the operation time is long, especially in regards to using local anesthesia, the patient may cough up sputum and other stimuli. If this occurs, a local spray of 1–2% lidocaine at regular intervals (30–40 minutes) is needed to strengthen the anesthesia. If there is local mucosal congestion, obvious swelling or local bleeding during ablation, 0.01% adrenaline or thrombin could be sprayed topically. If copious mucous secretions impact the operation vision, the catheter should be promptly used to clear airway secretions.
- The degree of expansion of the catheter electrode: the catheter electrode can present various degrees of expansion for different bronchial diameters. The assistants adjust the degree of expansion of the electrode through controlling the tension of the catheter handle. This is to ensure that the electrode matches the size of the bronchial cavity and lies closely to the wall for successful ablation. If the electrode is over-expanded in the small airway, the electrode may deform or damage the airway. If the electrode is under-expanded in the large airway, the activation will not be triggered. Therefore, the catheter holder should practice in vitro before operating in vivo in order to experience the different tensions associated with the corresponding degree of electrode when it expands.
- Electrode cleaning: due to activation of airway secretions and necrotic mucosal tissue coagulation attached to the electrode, then the visual field, the electrode conductivity and activation could be affected. Therefore, it should be promptly and carefully removed. Saline or wet gauze can be used to wash it. Damage to the temperature sensor on the tip of the catheter should be avoided. Note that alcohol or ice salt water cannot be used for cleaning.
- Team member: BT operation requires considerable skill and recognition of the tracheobronchial tree. The procedure requires a skilled doctor, an assistant to control the catheter, an assistant to record activations, an anesthesiologist and surgical nurses.
Postoperative complications management
Postoperative management
- Post-operative management
- Vital signs monitoring and clinical observation: some patients may experience an increase of short-term asthma symptoms (such as wheezing, shortness of breath, cough, chest tightness, etc.), upper and lower respiratory tract infection and fever. These patients can be given aerosol inhalation of bronchodilators or aerosolized corticosteroid treatment, or added with intravenous glucocorticoids and theophylline treatment. If a bacterial infection appears, antibiotic therapy should be given. Common post-operative symptoms are pharyngeal discomfort or sore throat, cough, sputum or sputum blood, and chest and back pain or headache. The symptoms disappear generally after 2 to 3 days while some patients may experience sputum about 1 to 2 weeks.
- pulmonary function test: the patients should be urged to perform PEF monitoring six hours after BT and should be closely monitored for the changes in condition for quick and effective treatment. Six hours after BT, the patient’s PEF should reach to or closely 80% of the individual’s pre-treatment level. That suggests that the patient’s post-operative asthma symptoms would be less likely to aggravate.
- Postural drainage: during post-operative nursing care, the patients need to be educated about the treatment site in regards to postural drainage and expectorant drugs in order to reduce lower respiratory tract mucous plugging, infection and help to avoid atelectasis.
- Discharge criteria
When the patient’s PEF value reaches more than 80% of the individual pre-operative level or pulmonary function FEV1%pred reaches more than 80% of the pre-operative BT level with no asthma symptoms, they could be discharged.
Prevention and treatment of complications
Short-term complications of BT include increased or worsened asthma-related respiratory symptoms (such as cough, chest tightness, wheezing, night awakening, etc.), lower respiratory tract infection, hemoptysis, atelectasis, pharyngitis, fever, headache and anxiety. Other complications such as pneumothorax, mucus plug, and lung abscess were also reported but are uncommon. Most of the adverse effects occurred the day after the surgery. Symptoms would alleviate within an average of 1 week automatically or by symptomatic treatment remission. In the clinical trials, there were no surgical-related fatal events reported. Based on published 5 years follow-up data, BT does not lead to negative long-term changes in the bronchial structure and long-term complications were not observed. Bronchoscopy or HRCT did not detect bronchiectasis, bronchial stenosis, or any airway injury. Common short-term complications and prevention are as follows:
- Asthma related symptoms increased: the damage of the local bronchial wall after treatment of BT will cause an increased inflammatory response, airway wall edema and increased secretions, which may lead to exacerbations of asthma and even acute attacks. As a result, patients may experience different degrees of coughing, wheezing, chest tightness and dyspnea. Close attention should be paid not only to the patients’ symptoms and signs, but also to the routine monitor of PEF and symptoms of exacerbation. The patient should be treated in accordance to the treatment principles regarding acute attacks of asthma.
- Lower respiratory tract infection: lower respiratory tract infection can be a common adverse reaction of BT treatment, which correlates with many factors as local airway injury, mucosal edema, increased secretions in relation to poor drainage, long-term use of corticosteroids, a progressive cough, purulent sputum (or increased sputum volume) and sputum bolt (with or without a fever). The operation should be performed with sterile/clean principles. Patients with severe lung function impairment, mucus hyper-secretion, or blockage of sputum drainage can be given antibiotics for short-term prevention and treatment. However, routine prophylactic use of antimicrobial drugs is not recommended.
- Bleeding: BT can cause airway wall injury and airway hemorrhage may occur during operation or after treatment. However, there is minimal bleeding and the blood in the sputum will resolve typically without intervention. During the operation, if there is significant bleeding, then the usual methods to control bleeding during bronchoscopy are deployed (diluted thrombin, cold-saline, balloon catheters, or epinephrine).
- Atelectasis: occasionally atelectasis occurs after BT. This is presumed to be related to the temporary increase of the bronchial mucosal inflammatory reaction, the edema of bronchial mucosa, and the blockage of the airway with a mucus plug. The full aspiration of airway secretions and necrosis during operation, postoperative drainage of sputum, body posture and the appropriate use of phlegm drugs or physical therapy to promote the removal of secretions can reduce the occurrence of pulmonary atelectasis. Mild pulmonary atelectasis could recover without special treatment. If the patient suffers dyspnea or hypoxia after the operation and atelectasis on chest imaging, then routine chest physiotherapy and mucous clearance techniques should be employed. If these fail to resolve the atelectasis, repeated bronchoscopy and aspiration of secretions may be needed.
- Pneumothorax and mediastinal emphysema: although rare, there has been case reports of these complications in patients with prior history of pulmonary tuberculosis and pleural adhesions. Thus, for patients with bronchial and pulmonary abnormalities, BT should be treated with caution.
- Anesthesia complications: there are a few complications in regards to local anesthesia. Severe complications of general anesthesia include hypoxemia, carbon dioxide retention, laryngeal and bronchial spasm, laryngeal edema, respiratory depression, aspiration pneumonia due to regurgitation, arrhythmia and even shock. Guidelines for the general management of sedation and anesthesia are published elsewhere.
Follow-up
- Follow up after a single surgery: following-up within 3 weeks after each BT treatment is important. Appointment visits and telephone follow-ups can urge patients to receive standardized drug therapy and improve their compliance so that they can maintain optimal control of their asthma and the next BT will be carried out on schedule. Any adverse events from the BT treatment should be observed, evaluated, and documented.
- Follow-up after BT treatments are complete: each patient should visit after the third session of BT. Follow-up every 1 to 3 months is recommended in order to evaluate the asthma control, adjust the treatment plan and observe the adverse events of BT. The efficacy of BT can be ensured and maintained through standardized follow-up visits after BT.
- Long term follow-up: it is recommended to follow-up 6 months, and 1, 3, 5 years after BT to access the long-term asthma control level and to evaluate the long-term efficacy and safety of BT.
Clinical benefit and risk
BT, as a supplement to the medical treatment of asthma, is a new choice for the individualized treatment for severe asthma (8,10-17). BT can reduce the mass and function of airway smooth muscles to improve asthma control, reduce drug use, improve patients’ quality of life, and reduce economic burdens. The effectiveness of BT has been demonstrated to be maintained for at least 5 years (11,14). BT is a safe and effective technique for the treatment of severe asthma, but there are still risks of adverse events in the short-term. No long-term complications and bronchial structural changes have been observed.
As the growth of BT in clinical practice continues, it is imperative to create a real-world clinical data set to further ensure the effectiveness, safety, and adaptability of BT (2,18,19). Along those lines, Chinese physicians have launched the “China Alair System Registry Study (NCT02206269)” and we look forward to the publication of the data.
Acknowledgements
Funding: This article was supported partially by Capital Clinical Application Research Foundation of Beijing Municipal Science and Technology Committee (2015-BKJ-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lin JT. Moving forward to improve the diagnosis and treatment of refractory asthma. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:561-2. [PubMed]
- Lin J, Qiu R. Pay attention to study on the appropriate populations and mechanism of bronchial thermoplasty. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:161-3. [PubMed]
- Nong Y, Su N, Lin J, et al. Effectiveness and safety of bronchial thermoplasty in patients with severe asthma. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:177-82. [PubMed]
- Zhang Q, Zhang X, Xie J, et al. Bronchial thermoplasty in the treatment of severe asthma. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:183-8. [PubMed]
- Qiu R, Lin J. Progressing of bronchial thermoplasty in asthma. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:213-7. [PubMed]
- Su N. Perioperative management of bronchial thermolpasty for asthma. Zhonghua Jie He He Hu Xi Za Zhi 2015;38:883-5. [PubMed]
- Li S. Standardized management of bronchial thermoplasty. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:166-8. [PubMed]
- Sheshadri A, Castro M, Chen A. Bronchial thermoplasty: a novel therapy for severe asthma. Clin Chest Med 2013;34:437-44. [Crossref] [PubMed]
- Cox G, Miller JD, McWilliams A, et al. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965-9. [Crossref] [PubMed]
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327-37. [Crossref] [PubMed]
- Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011;11:8. [Crossref] [PubMed]
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116-24. [Crossref] [PubMed]
- Castro M, Rubin A, Laviolette M, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011;107:65-70. [Crossref] [PubMed]
- Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013;132:1295-302. [Crossref] [PubMed]
- Berair R, Brightling CE. Asthma therapy and its effect on airway remodelling. Drugs 2014;74:1345-69. [Crossref] [PubMed]
- Kane B, Fowler SJ, Niven R. Refractory asthma - beyond step 5, the role of new and emerging adjuvant therapies. Chron Respir Dis 2015;12:69-77. [Crossref] [PubMed]
- Sheshadri A, McKenzie M, Castro M. Critical review of bronchial thermoplasty: where should it fit into asthma therapy? Curr Allergy Asthma Rep 2014;14:470. [Crossref] [PubMed]
- Bicknell S, Chaudhuri R, Lee N, et al. Effectiveness of bronchial thermoplasty in severe asthma in 'real life' patients compared with those recruited to clinical trials in the same centre. Ther Adv Respir Dis 2015;9:267-71. [Crossref] [PubMed]
- Lin J, Zhao Q. Diagnosis, evaluation and individualized treatment of severe asthma. Zhonghua Yi Xue Za Zhi 2015;95:3088-90. [PubMed]


