Diagnostic accuracy of tumor markers for malignant pleural effusion: a derivation and validation study
Introduction
Malignant pleural effusion (MPE) is frequently observed in some malignancies, implying systemic spread of cancer and reduction of life expectancy and quality (1). The initial diagnostic approaches to differentiation between benign pleural effusions (BPEs) and MPEs include thoracocentesis and cytological, histological and biochemical examinations; however, the sensitivity of these non-invasive techniques is only 40–70% (2). Many studies have investigated the usefulness of a number of tumor markers (TMs), including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, CA 15-3, and CA 19-9, in pleural fluid for the diagnosis of MPE (3). Our previous meta-analyses indicated that current evidence does not recommend using a single TM for the diagnosis of MPE; a combination of two or more TMs seemingly is more sensitive (4,5). We, along with others, have demonstrated that the concentration ratios between pleural effusion (PE) and serum (PE/serum ratio) of several TMs show better sensitivity with specificity than a single determination for PE (6-8). However, a majority of the published studies are single center designed, which lack validation. Our aims were to test the effectiveness of simultaneous determination of CEA, CA 125, CA 15-3, and CA 19-9 in PE, serum and the PE/serum ratio in diagnosing MPE; and especially, to evaluate the diagnostic accuracy of the ∆(PE–serum) for MPE.
Methods
Study populations
Beijing cohort and Wuhan cohort were chosen as derivation and validation randomly. All adult patients with undiagnosed PEs admitted to the Department of Respiratory and Critical Care Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing (derivation) and determined as necessary by the attending physicians were enrolled in this study between January 2013 and June 2015. Ultimately, consecutive patients with the establishment of a definite cause of PE were included. This population allowed the evaluation of the diagnostic accuracy of TMs for MPE, which was subsequently validated in a separate cohort of consecutive patients with PE who were recruited during the same period from the Department of Respiratory and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan (validation). The patients with a cytologically negative PE but a history of malignancy or subsequent diagnosis of malignancy were not included in the study. Additionally, PEs without a definite known cause were excluded due to the potential that the PE was malignant but missed by cytology.
The study was approved by the ethics committees of both participating institutions, and all study participants provided written informed consent. The study outcomes would not affect the future management of the patients. The corresponding authors vouch for the accuracy and completeness of the data and analysis as well as the fidelity of the study to technological and biostatistical protocols.
Diagnostic criteria
All MPE were obtained from lung cancer patients. A diagnosis of MPE was established by the demonstration of malignant cells in PE and/or on a pleural biopsy specimen. Tuberculosis PE (TPE) was diagnosed if Ziehl-Neelsen stains or Lowenstein-Jensen cultures of pleural fluid, sputum, or pleural biopsy specimens were positive or if granulomas were found in the parietal pleural biopsies. Parapneumonic PE was diagnosed as any PE associated with bacterial pneumonia, lung abscess, or bronchiectasis, and an empyema was defined when pus was present within the pleural space. The presence of heart failure was based on well-established diagnostic criteria, with transudative PE classified by Light’s criteria (9).
Sample processing
PEs were collected via diagnostic thoracentesis before patients received any therapy. They were collected in sterile tubes without anticoagulant and rapidly transferred to our laboratory with a blood sample obtained simultaneously from the same patient. PE and blood samples were centrifuged at 1,500 rpm for 10 min at 4 °C, and the supernatants were aliquoted and stored at −80 °C awaiting analysis of TMs. Each aliquot was used only once to prevent enzyme activation due to freeze-thawing processes.
TM assays
The concentrations of CEA (Abbott Ireland Diagnostics Division, Sligo, Ireland), CA 125 (Abott Laboratories, Malvern, PA, USA), CA 15-3 (Abott Laboratories, Malvern, PA, USA) and CA 19-9 (Abott Laboratories, Malvern, PA, USA) in PEs and sera were measured using chemiluminescent microparticle immunoassay technology according to the manufacturer’s protocols. Briefly, a 10–30 µL sample and a 50 µL antibody coated paramagnetic particle were incubated for 18 min. After washing, 50 µL of antibody acridinium–labeled conjugate was added and incubated for 4 min to create a reaction mixture. Following another wash cycle, pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units by the i2000 Optical System (Abbott, ARCHITECT Corporation, PA, USA). All assays were performed on coded samples by investigators who were unaware of the patient’s diagnosis in a single laboratory analysis. The minimum detectable concentration of CEA, CA 125, CA 15-3, and CA 19-9 were 0.50 ng/mL, 1.0 U/mL, 0.5 U/mL, and 2 U/mL, respectively. When a specimen’s value exceeded the measurement range for the reagent kits, the specimen was diluted using the manual dilution procedure. All samples were assayed in duplicate.
All TM measurements in blinded samples were performed one time by one trained technician at the Department of Clinical Examination, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan; and the results were then unblinded and analyzed by our investigators.
Statistical analysis
The concentrations of TMs are presented as medians (25th to 75th percentiles) because these data were normally distributed as determined by the Kolmogorov-Smirnov test. Changes of TMs in PE were adjusted for the serum by calculating the ∆(PE–serum) of each TM. Parametric tests were used because TMs data were normally distributed as determined by a normality test. Comparisons of TM data between MPEs and BPEs were performed using the Mann–Whitney U test. Comparisons of data in PEs and in corresponding sera were conducted using paired t-tests.
Receiver operating characteristics (ROCs) curves were drawn and the areas under the curve (AUC) were calculated to determine the diagnostic value of the concentrations of each marker in PE, serum, the PE/serum ratio as well as the ∆(PE–serum) values (10,11); the AUCs were compared using the Hanley and McNeil procedure (10). Optimum cut-off values were defined based on their highest diagnostic accuracy according to the ROC curves. Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive predictive value (PPV), and negative predictive value (NPV) (11) were calculated for each TM and for the combination of TMs. The parameters of diagnostic accuracy are shown together with their 95% confidence intervals (CI). Analysis was completed with SPSS Version 18.0 Statistical Software (Chicago, IL, USA), and P<0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
The present study, which consists of derivation and validation studies, enrolled 327 patients recruited from 2013 to 2015 in China. The characteristics of the included patients are summarized in Table 1. In the Beijing cohort (derivation cohort), there were 174 patients, including 67 MPEs and 107 BPEs. Distribution of histological diagnoses in the MPE group was as follows: 50 lung adenocarcinoma patients, 3 squamous cell lung carcinoma patients, 2 small cell lung carcinoma patients, and 12 undetermined lung cancer patients. The etiologic diagnoses of the BPE group were as follows: 61 tuberculosis patients, 19 parapneumonic patients, 4 heart failure patients and 23 miscellaneous patients.

Full table
Of the 153 PE patients in the Wuhan cohort (validation cohort), 29 had lung adenocarcinoma, 4 had squamous cell lung carcinoma, 4 had small cell lung carcinoma, 15 had undetermined lung cancer, 78 had tuberculosis, 8 had parapneumonic effusion, 13 had heart failure and 2 had miscellaneous BPE.
Concentrations of TMs in PEs
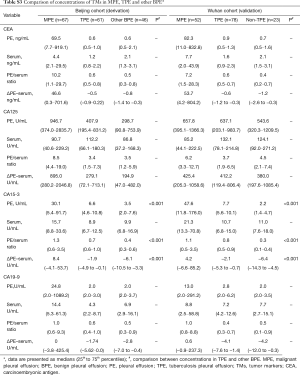
The PE concentrations of CEA, CA 125, CA 15-3, and CA 19-9 in MPE patients were all significantly higher than the concentrations in BPE patients (all P<0.001) in the Beijing cohort and the Wuhan cohort. The concentrations of four TMs were much higher in MPE than in the corresponding serum and BPE (P<0.001) in both cohorts. The concentrations of CEA, CA 15-3, and CA 19-9, but not CA 125, in sera in MPE patients were all significantly higher than those in BPE patients (all P<0.001) in the Beijing cohort and the Wuhan cohort. Additionally, a difference emerged from both PE/serum ratios and the ∆(PE–serum) values that were much higher in MPE than in BPE for CEA, CA 15-3, and CA 19-9 (all P<0.001), but not for CA 125 (Table 2).
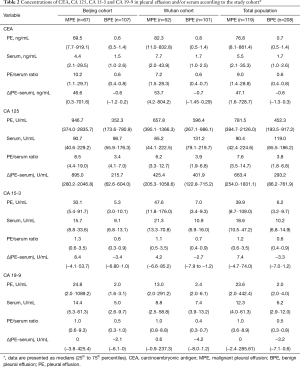
Full table
Diagnostic values of TMs
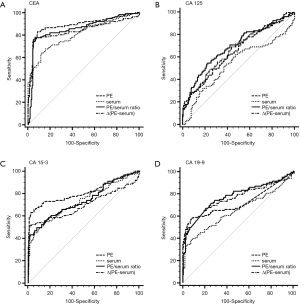
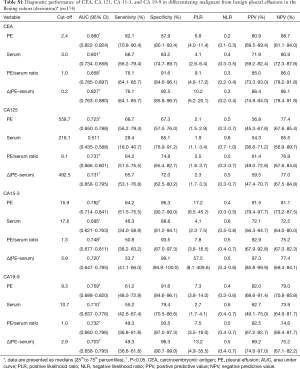
The capacity of TMs to differentiate malignant from benign PE was assessed with ROC curve analyses (Table 3, Figure 1, Tables S1,S2, Figures S1,S2). CEA and CA 15-3 could discriminate MPE from BPE in PE, serum, the PE/serum ratio and the ∆(PE–serum) value in the Beijing cohort, the Wuhan cohort and in the combined population (P<0.05). In the combined population, the diagnostic threshold provided by the ROC analysis for CEA in PE, serum, the PE/serum ratio and the ∆(PE–serum) value was 2.42 ng/mL (AUC: 0.890), 3.54 ng/mL (AUC: 0.806), 1.1 (AUC: 0.862), and 0.4 ng/mL (AUC: 0.849), respectively; and CA 15-3 was 17.8 U/mL (AUC: 0.813), 17.6 U/mL (AUC: 0.731), 1.3 (AUC: 0.738), and 5.9 U/mL (AUC: 0.698), respectively.
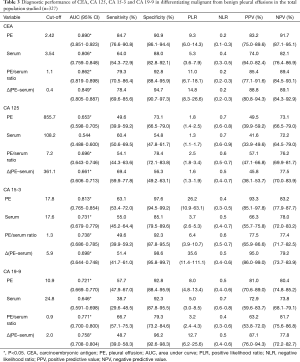
Full table
Full table
Full table
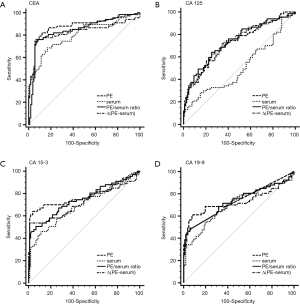
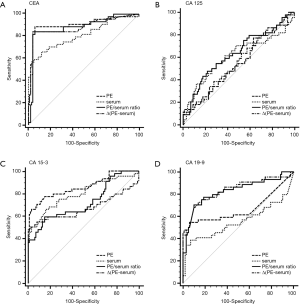
Concentrations of CEA and CA 15-3 were stable, and the trend of best diagnostic parameters was consistent and similar in Beijing, Wuhan and the combined population in sensitivity, specificity, PLR, NLR, PPV and NPV. In the combined population, higher diagnostic accuracy (0.890) of CEA was shown by using PE (sensitivity, 84.7%; specificity, 90.9%), the PE/serum ratio (AUC, 0.862; sensitivity, 79.3%; specificity, 92.8%) and the ∆(PE–serum) (AUC, 0.849; sensitivity, 78.4%; specificity, 94.7%). Additionally, the greatest diagnostic accuracy (0.813) of CA 15-3 was shown by using PE (sensitivity, 63.1%; specificity, 97.6%). With the greatest cut-off value, the highest specificity was presented using the ∆(PE–serum): 94.7% specificity in CEA (sensitivity, 78.4%; PLR, 14.8; NLR, 0.2; PPV, 88.8%; NPV, 89.1%) and 98.6% specificity in CA 15-3 (sensitivity, 51.4%; PLR, 35.6; NLR, 0.5; PPV, 95.0%; NPV, 79.2%).
Although CA 125 and CA 19-9 could discriminate MPE from BPE in PE, serum, the PE/serum ratio and the ∆(PE–serum) value in the combined population (Table 3), the concentration of CA 125 in PE and serum as well as cut-off values differed greatly between the two cohorts, and the trend of best diagnostic parameters was in disaccord between Beijing, Wuhan and the combined population. Similar inconsistent data were also observed in CA 19-9, except for data in the PE/serum ratio (Table 2, Tables S1,S2).
For each TM to obtain a specificity of 100% in the total population, the sensitivity, NLR and NPV are shown in Table 4. The highest sensitivity (37.8%) using serum was presented in CEA, with 19.8% sensitivity in PE and 18.0% sensitivity in the ∆(PE–serum). For CA 15-3, the sensitivity was 32.4% in PE, 15.3% in the PE/serum ratio and 25.2% in the ∆(PE–serum).
Full table
Discussion
The diagnosis of MPE is a frequent problem in clinical practice, especially considering different etiologies of PEs, the treatments options and prognoses involved. Approximately 30–60% of MPEs have negative thoracentesis with cytopathologic study of the pleural fluid. However, conventional methods have been shown to have lower sensitivity and are inadequate (12). Although thoracoscopy and thoracotomy present approximately 90% diagnostic sensitivity for malignant patients (13), they are expensive and have a risk of death (14). The use of TMs offers the potential for a cost-effective and minimally invasive alternative in the diagnosis of pleural malignancy. Although several TMs have been repeatedly studied, a problem of insufficient overall diagnostic accuracy has consistently been encountered.
In our previous retrospective analysis, we investigated the association between the levels of CEA, CA 125, CA 15-3 and CA 19-9 in patients with BPE and MPE and the combined detection of TMs to diagnose etiology of PE (8). After reviewing the literature, we discovered that a majority of the published studies are single centered with no validated cohort. In the present study, we performed the first derivation and validation study in China to investigate diagnosis efficacy of CEA, CA 125, CA 15-3 and CA 19-9 in serum, PE, PE/serum and the ∆(PE–serum) in patients with BPE or MPE. Our results confirmed that TMs are useful tools in the differential diagnosis etiologies of effusions, with significantly higher concentrations in patients with MPE than in those with BPE (15). Additionally, we have shown that the sensitivity and specificity of each of the TMs for diagnose MPE. CEA and CA 15-3 are the two validated TMs that have performed consistently in this derivation and validation study. The present study was to assess the diagnostic value of TMs to differentiate between MPE and BPE other than MPE and a specific diseases such as tuberculosis. However, we also have compared the concentrations of TMs in groups of TPE and other BPE to find whether it’s necessary to compare TPE and other BPE separately (Table S3). The results show that there are no differences in concentrations of CEA, CA 125 and CA 19-9 in TPE and other BPE in Beijing cohort (derivation) and Wuhan cohort (validation). Although there are statistical differences in TPE and BPE in level of CA 15-3, these concentrations are far from that in MPE. Therefore, we calculated TPE and other BPE together.
Full table
Compared to the other three TMs, CEA has the greatest diagnostic accuracy to differentiate pleural malignancy, which is in accordance with a previous study (16). In the total population, the AUCs in the ROC analysis for PE, serum, PE/serum ratio and ∆(PE–serum) were 0.890, 0.806, 0.862 and 0.849, respectively. These parameters were similar in the Beijing and Wuhan cohorts, and they appear to have highly consistent performances in diagnosis. In the combined population, a higher accurate performance was shown, for example, 84.7% sensitivity and 90.9% specificity found in PE (cut-off: 2.42), 79.3% sensitivity and 92.8% specificity found in the PE/serum ratio (cut-off: 1.1), and 78.4% sensitivity and 94.7% specificity in the ∆(PE–serum) (cut-off: 0.4). CEA is a glycoprotein component of the glycocalyx of the epithelium that is expressed in various tumors especially those of epithelial origin. The application of a PE/serum ratio >1.1 could differentiate pleural malignancy, and it is suggested that an increased CEA level in MPE may be caused more by a more direct mechanism such as pleural invasion.
CA 15-3 is expressed in many normal and malignant tissues, including breast, lung and ovarian cancers, and its secretion is increased in the presence of a tumor. CA 15-3 is another TM with consistent performance validated in our two cohorts. In the total population, the specificity of PE samples was 97.6% indicating a potential role for CA 15-3 analysis in the confirmation of MPE. In contrast with the high specificity, the sensitivity of PE samples for CA 15-3 was only 63.1%, which is insufficient to exclude MPE. A specificity of 98.6% in the ∆(PE–serum) with a sensitivity of 51.4% also showed the same diagnostic performance. The results of one published meta-analysis, including 21 studies with a total of 2,861 cases, were similar to our study and showed that the sensitivity and specificity of CA 15-3 in the diagnosis of MPE were 0.58 (95% CI: 0.56–0.61) and 0.91 (95% CI, 0.90–0.93) (17). However, we suggest that CA 15-3 alone is not recommended due to its limited sensitivity.
CA 125 is particularly useful in diagnosing and detecting the recurrence of gynecologic tumors (18,19); this marker is also observed in patients with lung, breast and gastrointestinal tract cancer. Evaluated CA 19-9 is often found in patients with gastrointestinal tumors. In the present study, the levels of CA 125 and CA 19-9 were not similar in the Beijing and Wuhan populations. Further, the main problem in differential diagnosis is the cut-off value. For example, the cut-off value of CA 125 in PE, serum, and the ∆(PE–serum) ranges from 558.7 to 383.9 U/mL, 216.1 to 91.4 U/mL and 492.5 to 2765.3 U/mL in the Beijing and Wuhan populations, respectively. The cut-off value of CA 19-9 in PE, serum, and the ∆(PE–serum) ranges from 9.3 to 11.1 U/mL, 10.7 to 23.5 U/mL and 2.9 to −0.6 U/mL in the Beijing and Wuhan populations, respectively. Reasons to explain these discrepancies may be related to tumor heterogeneity and other known or unknown metastases. Because data for CA 125 and CA 19-9 are inconsistent between the two cohorts, this indicates that CA 125 and CA 19-9 may be not appropriate to diagnose the etiology of PE.
To the best of our knowledge, this is the first study to use the ∆(PE–serum) to diagnose MPE. Interestingly, this parameter is very useful to differentiate MPE. In the total population using CEA the sensitivity and specificity was 0.78 (95% CI, 0.70–0.86) and 0.95 (95% CI, 0.91–0.97) for the ∆(PE–serum) compared with 0.85 (95% CI, 0.77–0.91) and 0.91 (95% CI, 0.86–0.94) for PE. For CA 15-3, sensitivity was 0.51 (95% CI, 0.42–0.61) and specificity was 0.99 (95% CI, 0.96–1.00) in the ∆(PE–serum) compared with the parameters for PE with a sensitivity of 0.63 (95% CI, 0.53–0.72) and a specificity of 0.98 (95% CI, 0.95–1.00). With a specificity of 100%, the diagnosis performance of CEA in the ∆(PE–serum) was similar to that of PE. For CA 15-3, with a specificity of 100%, the performance of the ∆(PE–serum) was also better than the value of serum and only next to the value of PE to diagnose the etiology of MPE.
The present study demonstrated that TMs are useful in the differential diagnosis of MPE and BPE. After derivation and validation, we concluded that compared to CA 125 and CA 19-9, CEA and CA 15-3 are more reliable to perform diagnostic tests. The use of the ∆(PE–serum) in TMs, such as CEA and CA 15-3, may improve the sensitivity and specificity of the diagnosis etiology of PE.
Acknowledgements
We thank Miss. Fang Wang at the department of clinical examination, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, for excellent technical assistance with the measurement of TMs. We also thank physicians and nurses at the participating departments for inclusion of patients in the present study. We also thank the patients for their participation in this study.
Funding: This work was supported in part by grants from National Natural Science Foundation of China (No. 91442109, No. 31470883, and No. 81470274), in part by the Beijing Municipal Administration of Hospitals’ Mission Plan (No. SML20150301), and in part by Chinese Ministry of Science and Technology for the establishment of GCP evaluation system in respiratory diseases (2014ZX09303302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committees of Beijing Chao-Yang Hospital (No. 2012-Ke-53) and Union Hospital (No. [2012]Lunshenzi(S092)), and all study participants provided written informed consent.
References
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [Crossref] [PubMed]
- Maskell NA, Butland RJ. Pleural Diseases Group, et al. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003;58 Suppl 2:ii8-17. [Crossref] [PubMed]
- Light RW. Tumor markers in undiagnosed pleural effusions. Chest 2004;126:1721-2. [Crossref] [PubMed]
- Shi HZ, Liang QL, Jiang J, et al. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology 2008;13:518-27. [Crossref] [PubMed]
- Liang QL, Shi HZ, Qin XJ, et al. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax 2008;63:35-41. [Crossref] [PubMed]
- Trapé J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumour Biol 2004;25:276-81. [Crossref] [PubMed]
- Trapé J, Molina R, Sant F, et al. Diagnostic accuracy of tumour markers in serous effusions: a validation study. Tumour Biol 2012;33:1661-8. [Crossref] [PubMed]
- Gu Y, Zhai K, Shi HZ. Clinical Value of Tumor Markers for Determining Cause of Pleural Effusion. Chin Med J (Engl) 2016;129:253-8. [Crossref] [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839-43. [Crossref] [PubMed]
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168-9. [Crossref] [PubMed]
- Sriram KB, Relan V, Clarke BE, et al. Diagnostic molecular biomarkers for malignant pleural effusions. Future Oncol 2011;7:737-52. [Crossref] [PubMed]
- Gourgoulianis KI, Hatzoglou CH, Molyvdas PA. The major route for absorption of fluid from the pleural space. Lymphology 2002;35:97-8. [PubMed]
- Maturu VN, Dhooria S, Bal A, et al. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: a single-center experience of 348 patients. J Bronchology Interv Pulmonol 2015;22:121-9. [Crossref] [PubMed]
- Shitrit D, Zingerman B, Shitrit AB, et al. Diagnostic value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literature. Oncologist 2005;10:501-7. [Crossref] [PubMed]
- Korczynski P, Krenke R, Safianowska A, et al. Diagnostic utility of pleural fluid and serum markers in differentiation between malignant and non-malignant pleural effusions. Eur J Med Res 2009;14 Suppl 4:128-33. [Crossref] [PubMed]
- Wu Q, Li M, Zhang S, et al. Clinical diagnostic utility of CA 15-3 for the diagnosis of malignant pleural effusion: A meta-analysis. Exp Ther Med 2015;9:232-8. [Crossref] [PubMed]
- Osman N, O'Leary N, Mulcahy E, et al. Correlation of serum CA125 with stage, grade and survival of patients with epithelial ovarian cancer at a single centre. Ir Med J 2008;101:245-7. [PubMed]
- Hirsch M, Duffy J, Davis CJ, et al. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG 2016;123:1761-8. [Crossref] [PubMed]


