The significance of pathological extracapsular vs. intracapsular lymph node involvement in patients with resectable esophageal cancer after neoadjuvant therapy
Introduction
The standard of care for locoregional esophageal cancer, regardless of histology, remains neoadjuvant chemotherapy or chemoradiation followed by an esophagectomy after restaging workup (1,2). The final pathological stage (ypTNM) determines if adjuvant therapy is needed. Common practice advocates the use of adjuvant chemotherapy or chemoradiation (if no preoperative radiation was administered) in cases of residual nodal disease (ypTxN+), whereas only surveillance imaging is warranted in node-negative patients (ypTxN0) (3).
The significance of extracapsular lymph node involvement (EC-LNI) versus intracapsular lymph node involvement (IC-LNI) in patients with esophageal cancer has long been a subject of investigation in the thoracic surgical literature (4-7). Although EC-LNI has been demonstrated to portend a worse survival compared to IC-LNI (4-7), this differentiation has yet to be incorporated into the staging system. Almost all studies assessing capsular invasion have been undertaken on patients with adenocarcinoma (AC) located in the distal esophagus or gastroesophageal junction who went directly to surgery. In fact, patients with locoregional disease treated with neoadjuvant chemotherapy or chemoradiation have not been a well-studied cohort in terms of the significance of EC-LNI versus IC-LNI, with only a single prior report in the literature (8). Patients undergoing neoadjuvant therapy prior to esophagectomy have been difficult to stratify because, until recently, the TNM classification system did not take such treatment into account; the Kaplan-Meier curves used to devise the staging system were based on patients undergoing surgery alone. This fact made assessment of post-neoadjuvant therapy esophagectomy patients difficult to interpret relative to overall and disease-free survival for various cancer stages.
The 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual, however, does consider patients undergoing neoadjuvant treatment prior to esophagectomy (ypTNM). This group of tumors is clinically quite relevant as most patients with resectable esophageal cancer, regardless of histology, undergo neoadjuvant therapy before proceeding to an esophagectomy (9,10). As a result, an important next step is to analyze whether the presence of EC-LNI after neoadjuvant treatment (ypN+EC-LNI) is associated with a worse prognosis than cases where invasion is limited to the nodes (ypN+IC-LNI).
This question was posed in a recent retrospective, multicenter European trial assessing the databases from six high-volume centers and including 1,505 eligible patients with either AC or squamous cell carcinoma (SCC) of the esophagus or esophagogastric junction who underwent an R0 resection following neoadjuvant chemoradiotherapy (nCRT) (11). The results of the trial determined the presence of ypN+EC-LNI after nCRT to be the strongest prognosticator of overall survival (OS) for SCC but not for AC. In addition, the study found that OS at 5 years was significantly worse for SCC in the presence of ypN+EC-LNI compared to ypN+IC-LNI or ypN0. For AC, no significant difference was seen in OS between ypN+EC-LNI and ypN+IC-LNI cohorts. Other important observations were that while SCC patients determined to be ypN1 had better overall survival in the absence of EC-LNI, ypN2 and ypN3 categories were found to have no survival difference between IC-LNI and EC-LNI groups, consistent with their poor overall prognosis regardless of extracapsular invasion. The authors suggested that ypN1 patients should be stratified based on EC-LNI status and tumor histology, with ypN+IC-LNI SCC carrying a favorable prognosis.
The previous literature has shown that patients undergoing esophagectomy without neoadjuvant treatment and found to have EC-LNI on pathologic assessment have the same 5-year OS (19%) regardless of histologic type as patients with systemic disease (M1) (12). On the other hand, patients who were pN1 with IC-LNI behaved in a similar fashion to those with pN0 disease. Once again, these studies were conducted on patients who were chemoradiotherapy naïve, and an extrapolation to patients undergoing neoadjuvant therapy and revealing ypN1 pathology cannot be assumed.
In light of the findings from the current study, an issue is whether patients with EC-LNI should be segregated and treated differently than those with IC-LNI. Pertinent questions are:
- What is the clinical significance of EC-LNI after neoadjuvant therapy?
- Should patients with ypN+EC-LNI tumors get adjuvant therapy while those with ypN1IC-LNI SCC should not?
- What impact does adjuvant therapy have on tumor recurrence and OS in both scenarios (ypN+EC-LNI and ypN1IC-LNI)?
- If ypN+ patients with EC-LNI truly behave as M1 patients, do they merit a surgical resection? If they are not treated with surgery, should they be committed to undergoing definitive chemoradiation?
- Is there an accurate way of identifying patients with ypN+EC-LNI disease before subjecting them to an esophagectomy?
To date, only one other study has been conducted on this subject (8). Patients with esophageal cancer (SCC or AC) treated with neoadjuvant chemotherapy with or without radiation were assessed for the significance of ypN+EC-LNI versus ypN+IC-LNI. Of the 704 patients analyzed, the 5-year OS was 62.7% for patients with N0 disease and 44.9% for patients who were ypN+ without EC-LNI, while it was only 14% if EC-LNI was identified (P<0.001). The median survival in patients with EC-LNI was 17 months (95% CI: 13–21), compared with 98 months (95% CI: 64–132) for those with IC-LNI only. Contrary to Depypere’s study, however, patients with SCC and AC were not segregated into distinct cohorts for analysis.
The diagnosis and stratification of residual nodal disease
A challenge that comes with prognosticating patients based on the presence or absence of nodal capsular invasion is the careful, reliable and reproducible evaluation of each resected lymph node by the pathologist. The time and effort spent in identifying EC-LNI versus IC-LNI has to be justified relative to the prognostic implications. This assessment would further burden pathologists to scrutinize each node to assess for capsular invasion in addition to determining the number of positive nodes and the classification into N1, N2, or N3 disease.
It would be interesting to investigate clinical predictors of persistent nodal disease after induction therapy for locoregional esophageal cancer, which may help in selecting patients most likely to benefit from surgical resection. Since studies have demonstrated AC to be associated with a higher incidence of EC-LNI than SCC, this fact potentially could explain why AC is comparatively less benefitted by neoadjuvant chemoradiation. Also, the location of the tumor or a pretreatment SUV perhaps could be added to a prediction model, which could eventually allow clinicians to estimate a patient’s prognosis (precision medicine).
Significance of nodal downstaging
Based on the potential for inducing a pathologic complete response (pCR), increased R0 resection rates, nodal downstaging, and improved OS, nCRT prior to esophagectomy has become the standard of care for locoregionally advanced esophageal or esophagogastric junction cancers in the United States and Europe (13,14). Most clinicians have adopted the policy of “hitting the tumor hard” with aggressive induction therapy before surgery (assuming the patient can tolerate it) in order to provide the best chance of cure or prolonging survival. Important questions remain, however, regarding the role of postoperative therapy in this setting, including the best adjuvant treatment regimen and whether it should be offered to all patients or only to those with residual disease. Currently, patients achieving a pCR typically are monitored with surveillance imaging alone. One consideration is whether patients found on their esophagectomy specimens to have lymph nodes lacking viable tumor cells but containing evidence of necrotic tumor should be considered as node-negative or node-positive. Neiman et al. questioned the significance of treatment-response lymph nodes from esophagectomy specimens and found that they carry a worse prognosis than those where lymph nodes are truly negative, suggesting that adjuvant therapy should be a consideration in the former cohort (15). This finding supports the argument that cases previously considered a pCR, though with evidence of necrotic tumor in lymph nodes, should not be considered ypN0, but indeed should be treated as ypN+. Further research will be needed to answer this question.
Summary
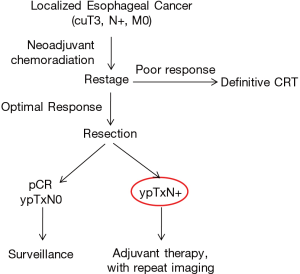
The implications of EC-LNI vs. IC-LNI in post-induction esophageal cancer patients undergoing esophagectomy remain controversial. Our strategy in this cohort is summarized in Figure 1. Patients with resectable clinical T3N0 or worse tumors are treated with neoadjuvant chemotherapy plus radiation (54 Gy). Those with adequate response as assessed on PET-CT (either stable disease or downstaging, without evidence of progression of disease) are counseled to undergo an esophagectomy. Patients with pCR and ypTxN0 pathology do not get treated with adjuvant therapy, while any nodal positivity seen on post-induction pathology is an indication for adjuvant therapy, assuming the patient is medically fit. While the best prognosis can be expected in those patients who had a pCR or were downstaged from their clinical stage, patients with residual nodal disease, particularly if SCC with EC-LNI, have the worst prognosis. Based on this most recent study, patients with SCC found to be ypTxN1IC-LNI may best be treated by observation alone, though further studies are necessary to prove this contention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Burt BM, Groth SS, Sada YH, et al. Utility of adjuvant chemotherapy After neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg 2017;266:297-304. [Crossref] [PubMed]
- Lagarde SM, ten Kate FJ, de Boer DJ, et al. Extracapsular lymph node involvement in node-positive patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Am J Surg Path 2006;30:171-6. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Extracapsular lymph node involvement is a negative prognostic factor in T3 adenocarcinoma of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg 2003;126:1121-8. [Crossref] [PubMed]
- Nafteux P, Lerut T, De Hertogh G, et al. Can extracapsular lymph node involvement be a tool to fine-tune pN1 for adenocarcinoma of the oesophagus and gastro-oesophageal junction in the Union Internationale contre le Cancer (UICC) TNM 7th edition? Eur J Cardiothorac Surg 2014;45:1001-10.
- Nafteux PR, Lerut AM, Moons J, et al. International multicenter study on the impact of extracapsular lymph node involvement in primary surgery adenocarcinoma of the esophagus on overall survival and staging systems. Ann Surg 2015;262:809-15; discussion 15-6. [Crossref] [PubMed]
- Lagarde SM, Navidi M, Gisbertz SS, et al. Prognostic impact of extracapsular lymph node involvement after neoadjuvant therapy and oesophagectomy. Br J Surg 2016;103:1658-64. [Crossref] [PubMed]
- Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:304-17.
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Depypere LP, Moons J, Mariette C, et al. Impact of extracapsular lymph node involvement after neoadjuvant chemoradiation therapy followed by surgery in carcinoma of the esophagus: a multicenter study. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Metzger R, Drebber U, Baldus SE, et al. Extracapsular lymph node involvement differs between squamous cell and adenocarcinoma of the esophagus. Ann Surg Oncol 2009;16:447-53. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Nieman DR, Peyre CG, Watson TJ, et al. Neoadjuvant treatment response in negative nodes is an important prognosticator after esophagectomy. Ann Thorac Surg 2015;99:277-83. [Crossref] [PubMed]


