Missed diagnosis of esophageal leiomyoma leading to esophagectomy: a case report and review of literatures
Introduction
Less than 10% of esophageal tumors are benign (1). Rare as the esophageal leiomyomas are, they are reported as the most common benign primary tumors of the esophagus, representing for approximately two thirds of the cases (2). Esophageal leiomyomas are usually found in middle-aged patients, and men are more frequently suffered than women by a ratio of 2:1. The size of esophageal leiomyoma is various, while approximate half of them are smaller than 5 cm in diameter (3). More than half of the patients are asymptomatic and found in incidentally. It is reported that the larger leiomyoma tends to have the greater possibility to show symptoms (4).
Transthoracic extramucosal blunt enucleation is considered the standard procedure of surgical treatment. However, it may not be suitable for giant leiomyoma due to the mucosal damage and potential sarcomatous change of the tumor.
Here we describe a case of esophageal leiomyoma who had been missed diagnosed for three years, and it developed as a consequence of giant one occupying the entire esophagus and finally received the esophagectomy.
Case presentation
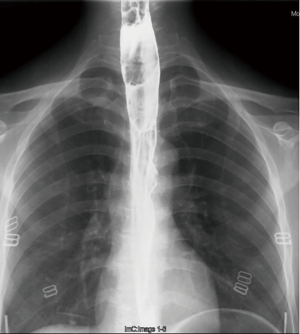
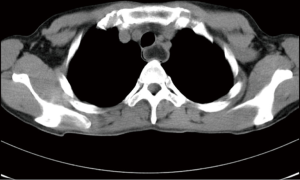
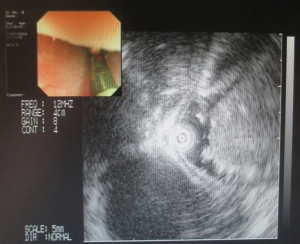
Three years ago, a 46-year-old woman was admitted with the complaint of repetitive eructation for 2 months, and nausea, vomiting, or weight loss were denied by patient. The patient had completed the examination of barium radiography showed a smooth filling defect in the contour esophageal lumen while no obvious disruption or broken of mucosa was found (Figure 1). At the same time, the examination of chest enhanced computed tomography (CT) showed a 6×3×2 cm3 irregular tissue in the upper thoracic esophagus (Figure 2). Endoscopy revealed a strip of submucosal bulge leading to lumen stricture (Figure 3). The endoscopic ultrasonography (EUS) showed a normal five-layered structure of the esophageal wall and the protrusive lesion was caused by a anterior mediastinal lesion (Figure 4). EUS instruments included an Olympus GIF-2T-240 double-cavity electronic gastroscope, Olympus MAJ drive systems with a high-frequency echoprobe, UM-DP12-25R miniature ultrasonic probes (MUP) with a frequency spectrum of 12 MHz, and a Daker WP-800 water pump (Olympus Medical System, Tokyo, Japan). From the perioperative examinations above, the initial diagnosis of mediastinal mass with esophageal compression was made. Video-assisted thoracoscopic surgery (VATS) with auxiliary mini-thoracotomy was performed subsequently. We found a lipoid mass originated from anterior mediastinum and had invaded the upper thoracic esophagus during the surgical exploration. After completing resection, histopathologic examination demonstrated the lipoid mass was thymolipoma tumor (Figure 5). Although the operation had brought considerable relief from eructation, the symptom still happened postoperative endoscopy showed the submucosal bulge was still existed. However, the patient refused any further examination and surgical treatment because of the inconsiderable symptom of the lesion.
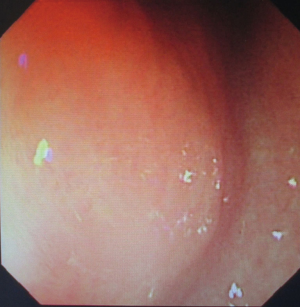
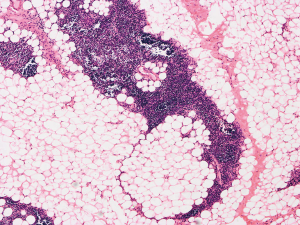
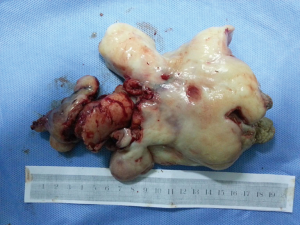
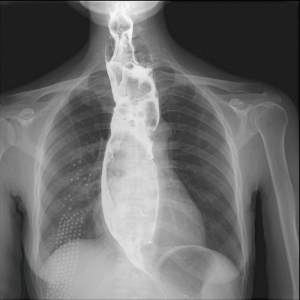
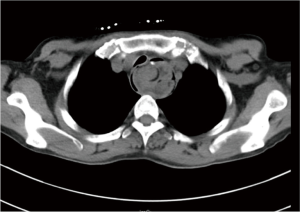
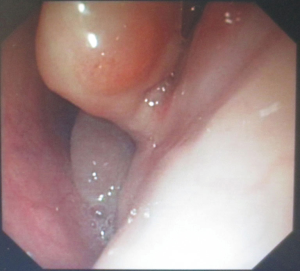
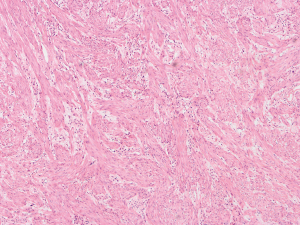
The patient was suggested completing chest CT scan and endoscopy after discharging. However, none examination had been performed over a course of three years until a progressive dysphagia occurred this year. A giant esophageal lesion was identified by barium radiography examination and an unusual filling defect and extreme dilatation of esophagus was found this time (Figure 6). At the same time, the chest enhanced CT showed an appropriate 20×9×8 cm3 pedunculated mass with fat density, heterogeneous enhancement and punctate calcifications (Figure 7), meanwhile, no enlarged lymph nodes or evidences of distance metastasis were found, and the surrounding tissues were compressed by the obviously dilated esophagus. The endoscopy examined a large intraluminal mass extending from the cervical esophagus until the cardia (Figure 8), and the examination was difficult to implement due to the sever esophageal retention. No endoscopic biopsy was performed. Esophageal leiomyoma was highly suspected and the patient had undergone the surgical exploration through right thoracotomy. During operation, an extremely dilated cervical and thoracic esophagus was found, and the esophageal wall became thinning and membranous. Esophageal lumen was opened for direct observation of the giant tumor. In macroscopic view, a 19×10×8 cm3 lobular and pedunculated mass, with a pedicle originated from the cervical esophagus and a maximum diameter of tumor near the cardia was 8 cm (Figure 9). Due to the limitation of the extensive esophageal lesion, open esophagectomy via a cervico-thoraco-abdominal approach was performed for curative intention instead of organ-sparing purpose. A gastric conduit through the posterior mediastinum was used to reconstruct the anastomosis with the cervical esophagus. The pathological report revealed well-defined submucosal smooth muscle tissue with mucoid degeneration and no mitotic activity or necrosis was present (Figure 10). It was reconfirmed by immunohistochemistry with a result of positive for desmin and smooth muscle actin (SMA) and negative for CD 34. The postoperative course was uneventful, and the patient was discharged subjectively free of complaints on the 9th postoperative day.
Discussion
Leiomyomas are preferably located in the lower third of the esophagus, and the reported proportions in the upper, middle, and lower third of the esophagus are 10%, 30%, and 60% respectively (1). Most of these tumors descend from the muscularis propria layer and typically present as oval or spherical. Occasionally, those arise from the muscularis mucosae may present as polypoid intraluminal tumors (5). Dysphagia is the most common complaint followed by retrosternal discomfort.
Esophageal leiomyomas with a diameter of more than 10cm are defined as “giant”. And a size of 19×10×8 cm3 leiomyoma in our case, which occupied the entire esophageal lumen, is less common. Another specialty for our case was the coincidence of thymolipoma and leiomyoma appeared simultaneously and the chief complaint of patient was eructation atypically. Furthermore, the upper esophageal muscular layer is predominately skeletal muscle and is seldom affected by leiomyoma which originates from smooth muscle cells.
Actually, esophageal leiomyoma is diagnosed from the examination of barium radiography, endoscopy, CT, and EUS. We hold the opinion that the biopsy is contraindicated. One of the reasons is that it may cause the fibrosis of the esophageal mucosa, increasing the risk of perforation and leading difficulties during surgical enucleation. Moreover, needle aspiration biopsy usually fails to identify the nature of the lesion for the lack of depth, especially for large leiomyoma as necrosis is often determined in the center (6-8).
As the modality of choice in diagnosing, EUS have failed to detect the esophageal intramural abnormality in our case. The reason we analyzed was the leiomyoma had already existed and EUS had a false-negative result in our patient’s initial report. It is reported that small esophageal leiomyomas are generally slow growing, and most of them are stable in lesion size over 3 years and more (9), while the large leiomyomas may grow rapidly and expand to 3 times or more in less than 3 years (3,9). Therefore, the giant leiomyoma found afterwards should be the smaller one three years ago. However, the esophageal compression of the coincident mediastinal lesion covered up the esophageal lesion and led to the false results by EUS.
In order to prevent such misdiagnosis from happening again in the future clinical treatment, we suggest the surgical therapy should be supported by intraoperative esophagoscopy, when the submucosal bulge is probably caused by compression of adjacent mediastinal structures or submucosal mass lesion itself. With the assistance of esophagoscopy, accurate pathological diagnosis can be made even small mucosal leakage during either resection or enucleation can be avoided as well (10).
It is generally believed that the surgical indications include persistent symptom, increased tumor size, mucosal ulcers, and simplification of surgical procedures. Considering that malignant transformation in leiomyoma is extremely rare (1) and the operative complications, asymptomatic patients with lesions smaller than 5 cm can be managed conservatively with regular endoscopic or CT scan follow-up (6,9). Transthoracic extramucosal blunt enucleation is the most frequently applied as the treatment option for small- to mid-sized (≤8 cm) esophageal leiomyoma (10). It can be completed by open surgery or minimally invasive approach. Since most leiomyomas are submucosal, localized and well encapsulated, which are conductive to take advantage of minimally invasive approach, we recommend that VATS should be the best choice. In case of large tumors (≥8 cm), enucleation by mean of VATS is difficult and demands experienced surgical skills. Meanwhile, the large muscular defect after enucleation is associated with an increased risk of diverticular-like bulging, mucosal perforation, and decreased muscular motility, even it can be buttressed with pedicled greater omental flap, pedicled pleural or muscular flaps (10,11). In addition, esophageal leiomyomas are slow-growing tumor and the large size implies high risk of potential sarcomatous change, therefore, esophageal resection should be considered as the safety and effective operative method.
In conclusion, the diagnosis of submucosal leiomyomas which was doubted by the results of endoscopy or CT scan might be missed because of the atypical symptoms, uncommon location, false results of EUS and coincident mediastinal occupying lesion. Intraoperative esophagoscopy or EUS is a valuable technique for diagnosing and simplifying the procedure of lesion resection or enucleation. Surgery is mandatory for midsize leiomyoma because of the rapid and intracavity growth tendency and the possibilities of malignant transformation. Meanwhile giant leiomyomas often need esophageal resection. In our hospital, the esophagectomy via a cervico-thoraco-abdominal esophageal resection is the recommended surgical procedure for giant extensive esophageal leiomyomas.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hatch GF 3rd, Wertheimer-Hatch L, Hatch KF, et al. Tumors of the esophagus. World J Surg 2000;24:401-11. [Crossref] [PubMed]
- Seremetis MG, Lyons WS, deGuzman VC, et al. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer 1976;38:2166-77. [Crossref] [PubMed]
- Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136-46. [Crossref] [PubMed]
- Jiang W, Rice TW, Goldblum JR. Esophageal leiomyoma: experience from a single institution. Dis Esophagus 2013;26:167-74. [Crossref] [PubMed]
- Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. Semin Thorac Cardiovasc Surg 2003;15:3-8. [Crossref] [PubMed]
- Iscan Y, Tunca F, Senyurek YG, et al. Thoracoscopic enucleation of a giant leiomyoma of the esophagus. Surg Laparosc Endosc Percutan Tech 2013;23:e32-4. [Crossref] [PubMed]
- Sun X, Wang J, Yang G. Surgical treatment of esophageal leiomyoma larger than 5 cm in diameter: A case report and review of the literature. J Thorac Dis 2012;4:323-6. [Crossref] [PubMed]
- Karagulle E, Akkaya D, Turk E, et al. Giant leiomyoma of the esophagus: a case report and review of the literature. Turk J Gastroenterol 2008;19:180-3. [PubMed]
- Xu GQ, Qian JJ, Chen MH, et al. Endoscopic ultrasonography for the diagnosis and selecting treatment of esophageal leiomyoma. J Gastroenterol Hepatol 2012;27:521-5. [Crossref] [PubMed]
- Rijcken E, Kersting CM, Senninger N, et al. Esophageal resection for giant leiomyoma: report of two cases and a review of the literature. Langenbecks Arch Surg 2009;394:623-9. [Crossref] [PubMed]
- Cheng BC, Chang S, Mao ZF, et al. Surgical treatment of giant esophageal leiomyoma. World J Gastroenterol 2005;11:4258-60. [Crossref] [PubMed]